
B-[4-(4-dibenzothienyl)phenyl]-boronic acid synthesis
- Product Name:B-[4-(4-dibenzothienyl)phenyl]-boronic acid
- CAS Number:1316275-42-9
- Molecular formula:C18H13BO2S
- Molecular Weight:304.17

121-43-7
380 suppliers
$13.00/25mL
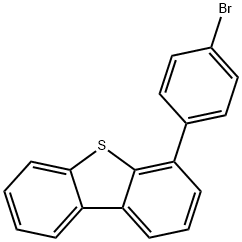
530402-77-8
61 suppliers
$60.00/5mg

7732-18-5
499 suppliers
$12.69/100ml
![B-[4-(4-dibenzothienyl)phenyl]-boronic acid](/CAS/20180629/GIF/1316275-42-9.gif)
1316275-42-9
18 suppliers
inquiry
Yield: 77%
Reaction Conditions:
Stage #1:4-(4-bromophenyl)dibenzo[b,d]thiophene with n-butyllithium in tetrahydrofuran;hexane at -78; for 1.5 h;Inert atmosphere;
Stage #2:Trimethyl borate in tetrahydrofuran;hexane at -78 - 20; for 20 h;Inert atmosphere;
Stage #3:water with hydrogenchloride in tetrahydrofuran;hexane
Steps:
3 Synthesis of 4-(dibenzothiophen-4-yl)phenylboronic acid
(0426) Into a 300-mL three-neck flask was put 6.7 g (20 mmol) of 4-(4-bromophenyl)dibenzothiophene that was synthesized in the step shown by Formula (C-1). After a nitrogen gas was made to flow continuously in the system, 200 mL of dehydrated tetrahydrofuran was added. This solution was cooled down to -78° C., 15 mL (24.0 mmol) of an n-butyllithium hexane solution (1.6 mol/L) was dropped with a syringe, and then, stirring was performed at the same temperature for 1.5 hours. After the stirring, 3 mL (26.8 mmol) of trimethyl borate was added at the same temperature and the resulting solution was stirred at room temperature for 20 hours. After the stirring, 90 mL of hydrochloric acid (1 mol/L) was added and the aqueous layer of the resulting mixture was subjected to extraction with ethyl acetate. The obtained solution of the extract and the organic layer were combined, and this mixture was washed with an aqueous solution of sodium hydrogen carbonate and saturated brine and dried with anhydrous magnesium sulfate. The resulting mixture was gravity-filtered and the filtrate was concentrated to give a pale yellow solid. The solid was washed with chloroform/hexane, whereby 4.0 g of a white solid of the target substance was obtained in a yield of 77%. The synthesis scheme of this step is shown in Formula (C-2).
References:
Semiconductor Energy Laboratory Co., Ltd.;Kawakami, Sachiko;ISHIGURO, Yoshimi;TAKAHASHI, Tatsuyoshi;HAMADA, Takao US2017/117487, 2017, A1 Location in patent:Paragraph 0426

5419-55-6
388 suppliers
$12.00/5g

530402-77-8
61 suppliers
$60.00/5mg
![B-[4-(4-dibenzothienyl)phenyl]-boronic acid](/CAS/20180629/GIF/1316275-42-9.gif)
1316275-42-9
18 suppliers
inquiry
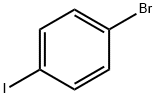
589-87-7
538 suppliers
$10.00/1g
![B-[4-(4-dibenzothienyl)phenyl]-boronic acid](/CAS/20180629/GIF/1316275-42-9.gif)
1316275-42-9
18 suppliers
inquiry
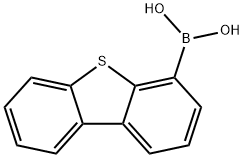
108847-20-7
341 suppliers
$11.19/1G
![B-[4-(4-dibenzothienyl)phenyl]-boronic acid](/CAS/20180629/GIF/1316275-42-9.gif)
1316275-42-9
18 suppliers
inquiry