Benzanthrone synthesis
- Product Name:Benzanthrone
- CAS Number:82-05-3
- Molecular formula:C17H10O
- Molecular Weight:230.26
Yield:82-05-3 98.8%
Reaction Conditions:
Stage #1:9,10-phenanthrenequinone with sulfuric acid;hydrogen;sodium dodecyl-sulfate in chlorobenzene under 10501.1 Torr;Heating;
Stage #2:glycerol with sulfuric acid in chlorobenzeneHeating;Reagent/catalyst;Pressure;
Steps:
1; 2; 3 Example 1
Prepare 21kg of anthraquinone (molecular weight 208.2, 101mol),Ruthenium carbon(With 5% palladium carbon)0.26kg,60% sulfuric acid 0.08kg,Phase transfer catalyst 210g sodium dodecyl sulfate,100kg of chlorobenzene,Put the above materials into a 600L pressure reactor,While breathing H2 gas,Make the pressure in the container reach 1400kPa,Stir for 6.5h at a temperature of 175 ;Cool the reaction solution to 110 ° C,After draining the remaining H2,Filtering the reaction liquid,Then wash with 30L chlorobenzene in batches,The filter cake is used for the recycling of the next batch of catalyst;Then the above chlorobenzene residual liquid and washing liquid,Pour into a 500L clean reactor,Then add glycerol with a concentration of 85%(glycerine) 20.6kg (molecular weight 92.1, 200mol),Heat to 105 ° C while stirring;Control the reaction temperature to 105 120 Add 35.8kg of 85% sulfuric acid(Molecular weight 98, 310mol),After the addition is complete,Continue stirring for 4.5h;After the reaction is completed, the reaction solution is cooled to below 80 ° C,Steam distillation in a 1000L distillation kettle,The vapor phase is condensed to the separator,The upper water returns to the kettle,The lower chlorobenzene collection chlorobenzene intermediate tank is applied to the next batch of reaction; after the chlorobenzene is completely distilled,filter,Collect mother liquor,Wash with hot water until neutral,Unloading,To get the phenanthrone filter cake,Further drying to obtain dry product benzanthrone, the purity is 98.5%, the yield is 98.8%;The mother liquor and the first washing water are collected,After treatment with 0.5 1.5% (mass percentage) activated carbon,Distillation and concentration under reduced pressure,Partially concentrated to sulfuric acid mass concentration of about 60% for the first batch of raw materials for reduction reaction recycling,The remaining part is concentrated to about 80% of sulfuric acid mass concentration,Then use fuming sulfuric acid (104%) or concentrated sulfuric acid (98%) to adjust the sulfuric acid mass concentration to 85% for the second step of condensation feeding.Other washing water is collected for environmental protection and centralized treatment.
References:
Jiangsu Yabang Dyeing Liao Co., Ltd.;Xiong Jun;Tang Xuebin;Zhou Jianbiao CN110668930, 2020, A Location in patent:Paragraph 0031-0043
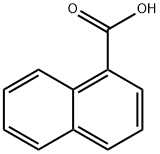
86-55-5
375 suppliers
$5.00/5g
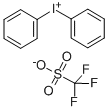
66003-76-7
158 suppliers
$6.00/250mg

82-05-3
170 suppliers
$11.00/25g
![7H-Benz[de]anthracene](/CAS/20200401/GIF/199-94-0.gif)
199-94-0
0 suppliers
inquiry

82-05-3
170 suppliers
$11.00/25g
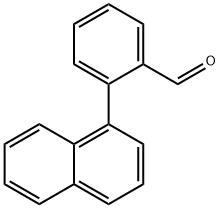
142598-69-4
26 suppliers
$165.00/250mg

82-05-3
170 suppliers
$11.00/25g
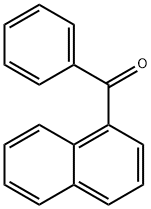
642-29-5
65 suppliers
$43.20/1g

82-05-3
170 suppliers
$11.00/25g