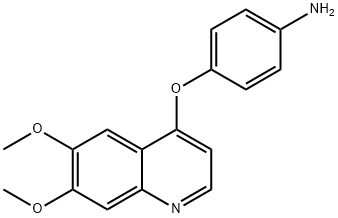
4-((6,7-Dimethoxyquinolin-4-yl)oxy)aniline synthesis
- Product Name:4-((6,7-Dimethoxyquinolin-4-yl)oxy)aniline
- CAS Number:190728-25-7
- Molecular formula:C17H16N2O3
- Molecular Weight:296.32
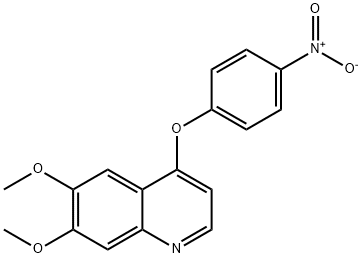
190728-24-6

190728-25-7
Step 2: Synthesis of 4-[(6,7-dimethoxyquinolin-4-yl)oxy]aniline (99) To a 1:1 MeOH/THF (50 mL) mixed solvent of 6,7-dimethoxy-4-(4-nitrophenoxy)quinoline (98) (0.25 g, 0.77 mmol) was added a solution of zinc powder (0.55 g, 8.4 mmol) and ammonium chloride (0.085 g, 1.6 mmol) in water (5 mL). The reaction mixture was heated to reflux for 2 hours. Upon completion of the reaction, the insoluble material was removed by diatomaceous earth filtration and the filtrate was concentrated under reduced pressure. The concentrated residue was dissolved in dichloromethane, washed sequentially with water and saturated saline, and the organic phase was dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give the crude product 99 (0.25 g, >100% yield), which can be used directly in the next reaction without further purification. Mass spectrometry (MS) data: m/z 297.1 ([M+H]+).
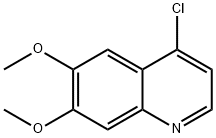
35654-56-9
363 suppliers
$13.00/1g
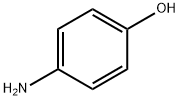
123-30-8
621 suppliers
$10.00/5g

190728-25-7
215 suppliers
$11.00/250mg
Yield:190728-25-7 90%
Reaction Conditions:
with sodium t-butanolate in N,N-dimethyl acetamide at -5 - 110;
Steps:
1; 2 Example 1 Preparation of Compound (II)
The compound (I) (500 g, 2.23 µM), 4-aminophenol (345 g, 3.16 µM) are added to a reaction in the bottle, adding DMA (3 L), lowering the temperature to 0 °C -5 °C, drop which is in butanol sodium (430 g) of DMA (2 L) suspension, then completing, heating up to 100 °C -110 °C, thermal insulation reaction 4 - 5 hours. (TLC monitoring the reaction is complete, Rf value 0.4 (dichloromethane: methanol=20:1)). Lowering the temperature to -5 °C -0 °C, adding ice water (10 L), stirring crystallization 15 - 16 hours. Filtering, cake a little water to wash, for 50 °C drying by blowing 15 - 16 hours to obtain light yellow solid 595 g, yield 90%, HPLC purity 99.7%
References:
Jiangsu Haosen Pharmaceutical Group Co., Ltd.;Fan Xingbao;Zhang Liang;Chen Anfeng;Zhou Bingcheng CN109836382, 2019, A Location in patent:Paragraph 0072-0077

190728-24-6
32 suppliers
inquiry

190728-25-7
215 suppliers
$11.00/250mg

35654-56-9
363 suppliers
$13.00/1g

190728-25-7
215 suppliers
$11.00/250mg
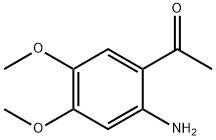
4101-30-8
142 suppliers
$7.00/250mg

190728-25-7
215 suppliers
$11.00/250mg
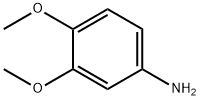
6315-89-5
314 suppliers
$7.00/10g

190728-25-7
215 suppliers
$11.00/250mg