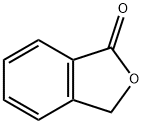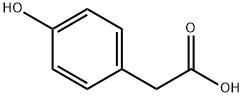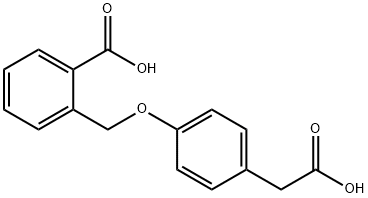
BENZENE ACETIC ACID, 4-[(2-CARBOXYPHENYL)METHOXY] synthesis
- Product Name:BENZENE ACETIC ACID, 4-[(2-CARBOXYPHENYL)METHOXY]
- CAS Number:55453-89-9
- Molecular formula:C16H14O5
- Molecular Weight:286.28
![Benzeneacetic acid, 4-[[2-(methoxycarbonyl)phenyl]methoxy]-, methyl ester](/CAS/20180808/GIF/1009378-92-0.gif)
1009378-92-0
![BENZENE ACETIC ACID, 4-[(2-CARBOXYPHENYL)METHOXY]](/CAS/GIF/55453-89-9.gif)
55453-89-9
The general procedure for the synthesis of 2-[(4-carboxymethylphenoxy)methyl]benzoic acid using the compound (CAS:1009378-92-0) as starting material was as follows: intermediate I (3.2 kg, 10.2 mol) and TEBA (34 g) were dissolved in ethanol and potassium hydroxide (1.2 kg, 21.4 mol) was added with stirring. The reaction mixture was heated to reflux for 3 hours. After completion of the reaction, the ethanol was removed by evaporation. Water (20 L) was added to the residue to dissolve it, followed by acidification with concentrated hydrochloric acid to pH 1-2, when a large amount of light yellow solid precipitated. The solid was collected by filtration and the filter cake was washed with water to neutral and dried to give Intermediate II (2.42 kg) as a light yellow solid in 83.1% yield.
![Benzeneacetic acid, 4-[[2-(methoxycarbonyl)phenyl]methoxy]-, methyl ester](/CAS/20180808/GIF/1009378-92-0.gif)
1009378-92-0
1 suppliers
inquiry
![BENZENE ACETIC ACID, 4-[(2-CARBOXYPHENYL)METHOXY]](/CAS/GIF/55453-89-9.gif)
55453-89-9
135 suppliers
$20.00/1g
Yield:55453-89-9 83.1%
Reaction Conditions:
Stage #1: 4-(2-methoxycarbonylbenzyloxy)phenyl acetic acidwith N-benzyl-N,N,N-triethylammonium chloride;potassium hydroxide in ethanol; for 3 h;Reflux;Large scale;
Stage #2: with hydrogenchloride in water; pH=1 - 2;Large scale;
Steps:
2.2 Example 2 Step 2. Preparation of intermediate II
The intermediate I (3.2 kg, 10 . 2mol) and 34gTEBA into ethanol, stirring, by adding potassium hydroxide (1.2 kg, 21 . 4mol), heating reflow 3h, evaporate the ethanol, the residue for adding 20L water-soluble. Acidification with concentrated HCl to pH=1-2, generating a large amount of a buff solid, filtered, the filter cake is washed with water to neutral, buff solid obtained after drying 2.42 kg, II as intermediate, yield: 83.1%.
References:
CN104262318,2016,B Location in patent:Paragraph 0053; 0054
2417-73-4
204 suppliers
$84.00/5g
![BENZENE ACETIC ACID, 4-[(2-CARBOXYPHENYL)METHOXY]](/CAS/GIF/55453-89-9.gif)
55453-89-9
135 suppliers
$20.00/1g
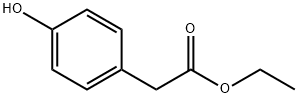
17138-28-2
210 suppliers
$14.00/1g
![BENZENE ACETIC ACID, 4-[(2-CARBOXYPHENYL)METHOXY]](/CAS/GIF/55453-89-9.gif)
55453-89-9
135 suppliers
$20.00/1g
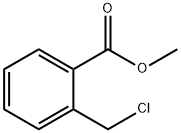
34040-62-5
103 suppliers
$25.00/1g
![BENZENE ACETIC ACID, 4-[(2-CARBOXYPHENYL)METHOXY]](/CAS/GIF/55453-89-9.gif)
55453-89-9
135 suppliers
$20.00/1g