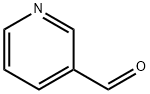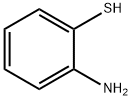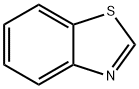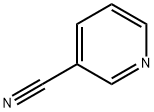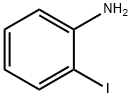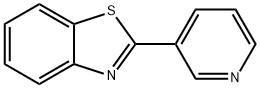
Benzothiazole, 2-(3-pyridinyl)- (9CI) synthesis
- Product Name:Benzothiazole, 2-(3-pyridinyl)- (9CI)
- CAS Number:2612-72-8
- Molecular formula:C12H8N2S
- Molecular Weight:212.27
Yield:2612-72-8 95%
Reaction Conditions:
with sulfated tungstate in neat (no solvent) at 20;Sonication;Green chemistry;
Steps:
General procedure for preparation of 2-substituted benzothiazole
General procedure: A mixture of 2-aminothiophenol (2 mmol) and aldehydes (2 mmol), and sulfated tungstate (10 wt%) was sonicated at 20 kHz frequency and 35W power at room temperature for desired times (monitored by TLC). After completion of the reaction as indicated on TLC, the reaction mixture was cooled at room temperature and the crude product was dissolved in ethyl acetate and the catalyst was isolated by simple filtration. It was then dried and reused for another reaction. The product in ethyl acetate was washed with water and excess solvent was removed under reduced pressure. The pure products 2-substituted benzothiazoles were obtained in 88-98% yield and there is no need to purify the products by recrystallization or column chromatography.
References:
Pise, Ashok S.;Ingale, Ajit P.;Dalvi, Navnath R. [Synthetic Communications,2021,vol. 51,# 23,p. 3629 - 3641] Location in patent:supporting information