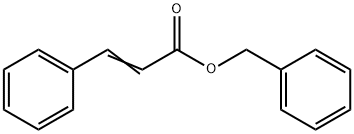
Benzyl cinnamate synthesis
- Product Name:Benzyl cinnamate
- CAS Number:103-41-3
- Molecular formula:C16H14O2
- Molecular Weight:238.28
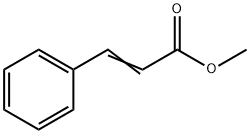
103-26-4
579 suppliers
$6.00/25g

100-51-6
1433 suppliers
$5.00/100g

103-41-3
381 suppliers
$5.00/250mg
Yield:103-41-3 96%
Reaction Conditions:
with iron(III)-acetylacetonate in n-heptane at 105; for 10 h;Inert atmosphere;
Steps:
Representative procedure for transesterification reactions catalyzed by Fe(acac)3 in the absence of Na2CO3
General procedure: To a dry 25 mL, bottomed flask equipped with a Dean-Stark trap containing a plug of 4Å molecular sieves (pellets) and topped with a reflux condenser was added of Fe(acac)3 ( 36 mg, 0.10 mmol, 5 mol%) and a solution of methyl bezoate (272 mg, 256 .L, 2.0 mmol), benzyl alcohol (216 mg, 208 .L, 2.0 mmol) and triphenyl methane (488 mg, 2 mmol, as internal standard) in heptane (20 mL). The mixture was heated to reflux (105 ° C) for an indicated time periods. After completion of the reaction as monitored by TLC, 1H NMR and GC, the reaction mixture was cooled to room temperature and the solvent was evaporated. The crude product was purified by column chromatography on silica gel to afforded benzyl benzoate 403 mg, 95% yield. The product obtained was characterized by 1H, 13C NMR, ESI-MS or GC-MS spectroscopic methods. The conversions of the products determined by GC are based on triphenyl methane as an internal standard and are response-corrected based on authentic samples.
References:
Weng, Shiue-Shien;Ke, Chih-Shueh;Chen, Fong-Kuang;Lyu, You-Fu;Lin, Guan-Ying [Tetrahedron,2011,vol. 67,# 9,p. 1640 - 1648] Location in patent:supporting information; experimental part
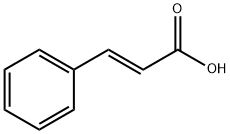
140-10-3
643 suppliers
$5.00/10g

100-51-6
1433 suppliers
$5.00/100g

103-41-3
381 suppliers
$5.00/250mg
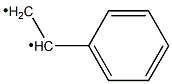
292638-84-7
0 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

100-51-6
1433 suppliers
$5.00/100g

103-41-3
381 suppliers
$5.00/250mg
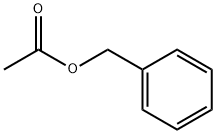
140-11-4
667 suppliers
$6.00/25g

100-52-7
970 suppliers
$17.30/2g

103-41-3
381 suppliers
$5.00/250mg

140-10-3
643 suppliers
$5.00/10g
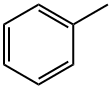
108-88-3
0 suppliers
$21.80/5 mL

103-41-3
381 suppliers
$5.00/250mg