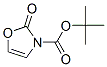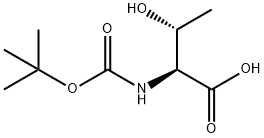
Boc-L-Threonine synthesis
- Product Name:Boc-L-Threonine
- CAS Number:2592-18-9
- Molecular formula:C9H17NO5
- Molecular Weight:219.24
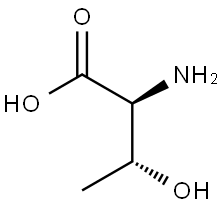
72-19-5

24424-99-5

2592-18-9
The general procedure for the synthesis of (2S,3R)-2-((tert-butoxycarbonyl)amino)-3-hydroxybutyric acid from L-threonine and di-tert-butyl dicarbonate was as follows: 1. To a methanol (5 mL) solution of L-threonine (400 mg, 3.36 mmol), a water (5 mL) solution of sodium bicarbonate (434 mg, 5.17 mmol) was added, followed by di-tert-butyl dicarbonate (1.07 g, 4.90 mmol). 2. The reaction mixture was stirred at room temperature for 3 days. 3. Upon completion of the reaction, the solvent was removed by vacuum distillation. 4. 4. The residue was diluted with water (20 mL) and washed with ether (2 x 20 mL). 5. The aqueous layer was acidified with saturated aqueous sodium bisulfite and then extracted with 2-methyltetrahydrofuran. 6. The organic layer was dried over anhydrous sodium sulfate and subsequently concentrated in vacuo to afford the title compound (2S,3R)-2-((tert-butoxycarbonyl)amino)-3-hydroxybutyric acid as a white solid (730 mg, 99% yield). 7. The product was characterized by 1H NMR (400 MHz, CDCl3) and LC-MS: 1H NMR δ 1.24 (d, 3H), 1.44 (s, 9H), 4.26 (d, 1H), 4.40 (d, 1H), 5.52 (d, 1H), 5.69 (br s, 1H); LCMS Rt = 1.68 min, MS m/z 218 [M + H]+.

72-19-5
762 suppliers
$4.76/25g

24424-99-5
869 suppliers
$13.50/25G

2592-18-9
323 suppliers
$5.00/1g
Yield:2592-18-9 99%
Reaction Conditions:
with sodium hydrogencarbonate in methanol;water at 20; for 72 h;
Steps:
52 Preparation 52
Preparation 52 N-Boc-L-threonine To a solution of L-threonine (400 mg, 3.36 mmol) in MeOH (5 mL) was added NaHCO3 (434 mg, 5.17 mmol) in water (5 mL) followed by Boc2O (1 .07 g, 4.90 mmol) and the reaction stirred at room temperature for 3 days. The solvent was removed in vacuo and the residue diluted with water (20 mL) and washed with ether (2 x 20 mL). The aqueous layer was acidified with saturated aqueous NaHSO solution and extracted with 2-MeTHF. The organic layer was dried over Na2SO and concentrated in vacuo to afford the title compound as a white solid (730 mg, 99%). 1H NMR (400MHz, CDCI3): δ ppm 1 .24 (d, 3H), 1 .44 (s, 9H), 4.26 (d, 1 H), 4.40 (d, 1 H), 5.52 (d, 1 H), 5.69 (br s, 1 H). LCMS Rt = 1 .68 minutes MS m/z 218 [M+H]+
References:
PFIZER INC.;BAGAL, Sharanjeet Kaur;BROWN, Alan Daniel;KEMP, Mark Ian;KLUTE, Wolfgang;MARRON, Brian Edward;MILLER, Duncan Charles;SKERRATT, Sarah Elizabeth;SUTO, Mark J.;WEST, Christopher William;MALET SANZ, Laia WO2013/114250, 2013, A1 Location in patent:Page/Page column 161; 162
15260-10-3
277 suppliers
$8.00/5g

2592-18-9
323 suppliers
$5.00/1g

72-19-5
762 suppliers
$4.76/25g

2592-18-9
323 suppliers
$5.00/1g
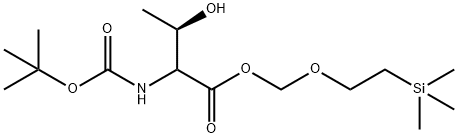
126587-71-1
1 suppliers
inquiry

2592-18-9
323 suppliers
$5.00/1g