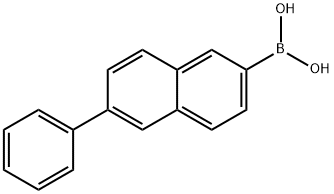
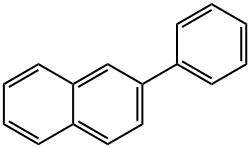
Boronic acid, (6-phenyl-2-naphthalenyl)- synthesis
- Product Name:Boronic acid, (6-phenyl-2-naphthalenyl)-
- CAS Number:876442-90-9
- Molecular formula:C16H13BO2
- Molecular Weight:248.08
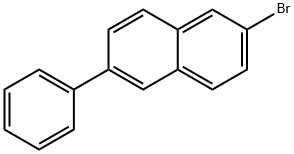
22082-94-6
27 suppliers
inquiry

876442-90-9
73 suppliers
$19.60/250mg
Yield:876442-90-9 55%
Reaction Conditions:
Stage #1: 6-phenyl-2-bromonaphthalenewith n-butyllithium in tetrahydrofuran;diethyl ether;hexane at -20; for 1 h;Inert atmosphere;
Stage #2: with Triisopropyl borate in tetrahydrofuran;diethyl ether;hexane at -60 - 20; for 16 h;Inert atmosphere;
Stage #3: with hydrogenchloride in tetrahydrofuran;diethyl ether;hexane;water at 20; for 1 h;
Steps:
5.2 [Synthesis Reference 5-2] Synthesis of 6-phenylnaphthalene-2-boronic acid
Under an argon gas atmosphere, a mixture of 100.0 g (353.1 mmol) of 2-bromo-6-phenylnaphthalene, 1.2 L of dehydrated THF and 1.2 L of dehydrated diethyl ether was cooled down to minus 20 degree C. Then, 280 ml (437 mmol) of hexane solution of 1.56M n-butyllithium was dropped into the mixture while the mixture was being stirred. The reaction mixture was further stirred at minus 20 degrees for 1 hour. The reaction mixture was cooled down to minus 60 degrees C., and 199.3 g (1.06 mol) of triisopropylborate was dropped into the mixture. The reaction mixture was warmed up and then stirred at room temperature for 16 hours. The reaction mixture was added with aqueous solution of hydrochloric acid and stirred at room temperature for 1 hour. After the reaction, the reaction mixture was added with toluene, and aqueous phase thereof was eliminated. Then, organic phase thereof was washed with water and dried with magnesium sulfate, and the solvent was distilled away under reduced pressure. By recrystallizing the obtained solid with hexane, 58.0 g of 6-phenylnaphthalene-2-boronic acid was obtained at an yield of 55%.
References:
US2010/331585,2010,A1 Location in patent:Page/Page column 97

121-43-7
384 suppliers
$14.00/25mL

22082-94-6
27 suppliers
inquiry

876442-90-9
73 suppliers
$19.60/250mg
130201-21-7
20 suppliers
inquiry

876442-90-9
73 suppliers
$19.60/250mg
13720-06-4
234 suppliers
$41.00/1g

876442-90-9
73 suppliers
$19.60/250mg
612-94-2
71 suppliers
$110.00/50mg

876442-90-9
73 suppliers
$19.60/250mg