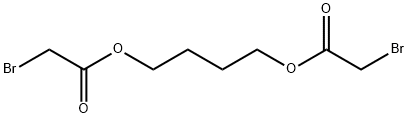
Bromoacetic acid 1,4-butanediyl ester synthesis
- Product Name:Bromoacetic acid 1,4-butanediyl ester
- CAS Number:67638-54-4
- Molecular formula:C8H12Br2O4
- Molecular Weight:331.99
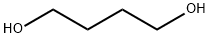
110-63-4
679 suppliers
$24.80/250g
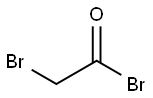
598-21-0
302 suppliers
$10.00/10g

67638-54-4
21 suppliers
$208.00/1g
Yield:67638-54-4 82%
Reaction Conditions:
in dichloromethane; for 20 h;Reflux;
Steps:
Synthesis of 4-bromoacetoxy-1-(S-glutathionyl)-acetoxy butane (4BAB)
The synthetic route to 4BAB is outlined in Figure 1. First, the starting di-ester bromoacetic acid 4-(2-bromo-acetoxy) butyl ester (BBE) was synthesized by dissolving 1.00 g (1.02 mL, 0.011 mol) of 1,4-butanediol in 50 mL of methylene chloride followed by addition of 4.44 g (1.92 mL, 0.022 mol) of bromoacetyl bromide, producing a yellow transparent solution. The solution was refluxed for 20 h and the solvent was removed in vacuo using a Buchi Rotavapor Re111 Evaporator yielding an orange tinted crystalline solid. The crystals were re-dissolved in a minimum amount of methanol and re-crystallized by the addition of water slowly until a white precipitate formed. The precipitate was vacuum filtered and air dried overnight. The crystals were a flaky white solid with an 82% percent yield and melting point of 60-62.5 C. The 1H NMR was consistent with the structure of BBE (d 1.75-1.79 (t, -O-CH2CH2-); d 3.83 (s, Br-CH2C(O)O-); d 4.19-4.23(t, C(O)OCH2-), Fig. S1). Four grams (12 mmol) of BBE was dissolved in 150 mL of methanol with stirring. A glutathione (GSH) solution was prepared by dissolving 0.8329 g (2.71 mmol) of GSH in 15 mL of degassed water and treating this solution with 1.1 mL of 5 M NaOH to bring the pH of the solution to 9.5. The GSH solution was added all at once to the BBE solution with vigorous stirring. After two minutes, formic acid was added until the pH of the solution was 3.5. The solvent was removed in vacuo and a white solid remained. Water, 20 mL, was added to the solid to dissolve 4BAB and any water-soluble impurities and 150 mL ofdiethyl ether was added to dissolve the excess BBE. The two layers from the filtrate were separated and the aqueous layer was savedand washed once more with 150 mL of ether. The aqueous layerwas filtered through an aqueous syringe filter tip and 4BAB was purified by on a Waters Delta 600 HPLC equipped with a Waters996 Photodiode Array Detector with a SymmetryPrep C18(7 lm 100 ? 19 150 mm) column (Waters) using a linear ABgradient from 5% to 60% B for 12.5 min and 60% to 100% B for2.5 min and a flow rate of 10 mL/min (where Solvent A was 0.1%aqueous TFA and Solvent B was 0.1% TFA in acetonitrile). The major peak at 9 min was collected and the product concentrated until 2 mL remained. This was flash frozen in an acetone/dry ice bathfor 15 min then lyophilized to dryness. Clear/white crystalsremained with a percent yield of 20%. The 1H NMR was acquiredon a ECX 400 MHz NMR (JEOL, Peabody, MA, USA) and MS analysiswas performed on a Apex IV Qh Ultra FT-ICR (Bruker Daltonics,Fremont, CA, USA). Both the 1H NMR (d 1.76-1.80 (m, -O-CH2CH2-);d 2.19-2.28 (m, Glu-CbH2); d 2.56-2.63 (m, Glu-CYH2); d 2.93-2.99 (q, Cys-CaHa); d 3.12-3.17 (q, Cys-CaHb); d 3.41-3.49(q, -S-CH2-C(O)); d 4.02 (s, -OC(O)-CH2-Br), d 4.05 (s, Gly-CH2);4.05-4.09 (t, Glu-CaH); d 4.21-4.27 (m, C(O)O-CH2-CH2-); d4.59-4.63 (q, Cys-CaH) and MS analysis are consistent with thestructure and theoretical mass of 4BAB (Supplemental Figs. S2-S4,respectively).
References:
Holewinski, Ronald J.;Creighton, Donald J. [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 13,p. 3301 - 3308]
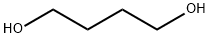
110-63-4
679 suppliers
$24.80/250g
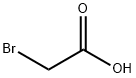
79-08-3
295 suppliers
$9.00/1g

67638-54-4
21 suppliers
$208.00/1g
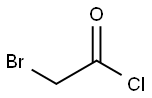
22118-09-8
171 suppliers
$21.21/10gm:
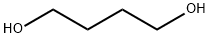
110-63-4
679 suppliers
$24.80/250g

67638-54-4
21 suppliers
$208.00/1g