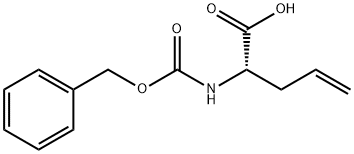
CBZ-ALPHA-ALLYL-L-GLY synthesis
- Product Name:CBZ-ALPHA-ALLYL-L-GLY
- CAS Number:78553-51-2
- Molecular formula:C13H15NO4
- Molecular Weight:249.26
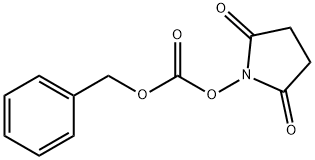
13139-17-8
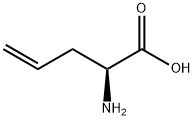
16338-48-0

78553-51-2
(S)-(-)-2-Amino-4-pentenoic acid (2.0 g) was suspended in dioxane (80 mL) at room temperature and triethylamine (4.3 g), water (2.0 mL) and N-(phenylmethoxycarbonyloxy)succinimide (9.1 g) were added sequentially. The reaction mixture was stirred overnight at room temperature and then concentrated under vacuum. Subsequently, the reaction mixture was diluted with saturated NaHCO3 solution and extracted with ether (3 x 80 mL). The basic aqueous layer was acidified to pH 2 with 2N HCl and extracted with EtOAc (3 x 80 mL). The EtOAc layers were combined, washed with brine, dried over anhydrous Na2SO4, filtered and the solvent evaporated. Finally, a viscous oily product (4.41 g, quantitative yield) was obtained by concentration from toluene (2 × 20 mL). The product was confirmed by 1H NMR (300 MHz, DMSO-d6): δ 2.31-2.50 (m, 2H), 4.00-4.06 (m, 1H), 5.03 (s, 2H), 5.05-5.12 (m, 2H), 5.71-5.84 (m, 1H), 7.35 (m, 5H), 7.53 (d, 1H), 12.57 (bs, 1H). Mass spectrometry analysis (APCI) showed m/z = 250 ([M+H]+).LC/MS analysis (Method A) showed a retention time of 2.30 min.

13139-17-8
522 suppliers
$6.00/25g

16338-48-0
261 suppliers
$11.00/250mg

78553-51-2
60 suppliers
$36.00/100mg
Yield:78553-51-2 100%
Reaction Conditions:
with water;triethylamine in 1,4-dioxane;
Steps:
2.a a. (2S)-2-{ F (BENZYLOXY) CARBONVLLAMINOLPENT-4-ENOIC acid (2a)
To a suspension of L-2-amino-4-pentenoic acid (2.0 g) in Dioxane (80 mL) at RT was added triethylamine (4. 3 g), water (2.0 mL), and N- (BENZYLOXYCARBONYLOXY) succinimide (9.1 g). The reaction mixture was stirred overnight at RT, concentrated in vacuo, diluted with saturated NaHCO3 and extracted with ether (3x). The basic aqueous layer was acidified to pH 2 with 2N HCl and extracted with EtOAc (3x). The combined EtOAc layer was washed with brine, dried (NA2S04), filtered and evaporated, then concentrated from Toluene (2x) to give a viscous oil (4. 51 g, QUANTITATIVE). LH NMR (300 MHz, DMSO-d6) 6 2.31-2. 50 (M, 2H), 4. 00-4. 06 (m, 1H), 5.03 (s, 2H), 5.05-5. 12 (m, 2H), 5.71-5. 84 (m, 1H), 7.35 (M, 5H), 7.53 (d, 1H), 12.57 (bs, 1H). MS APCI, m/z = 250 (M+1). LC/MS 2.30 min. , Method A.
References:
WO2004/80983,2004,A1 Location in patent:Page 47

16338-48-0
261 suppliers
$11.00/250mg

501-53-1
407 suppliers
$10.00/1g

78553-51-2
60 suppliers
$36.00/100mg
50299-14-4
25 suppliers
inquiry

501-53-1
407 suppliers
$10.00/1g

121786-40-1
6 suppliers
inquiry

78553-51-2
60 suppliers
$36.00/100mg

149117-86-2
0 suppliers
inquiry

50299-14-4
25 suppliers
inquiry

78553-51-2
60 suppliers
$36.00/100mg
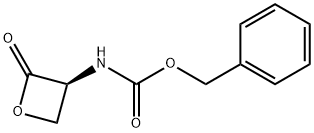
26054-60-4
117 suppliers
$91.00/250mg
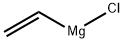
3536-96-7
204 suppliers
$67.00/100ml

78553-51-2
60 suppliers
$36.00/100mg