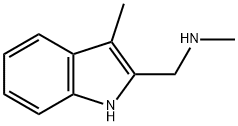
CHEMBRDG-BB 4010855 synthesis
- Product Name:CHEMBRDG-BB 4010855
- CAS Number:894852-67-6
- Molecular formula:C11H14N2
- Molecular Weight:174.24
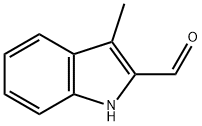
5257-24-9
65 suppliers
$73.00/500mg

74-89-5
0 suppliers
$13.44/25ML

894852-67-6
14 suppliers
$369.00/500mg
Yield:894852-67-6 71%
Reaction Conditions:
Stage #1: 2-formyl-3-methylindole;methylamine in methanol;ethanol; for 5 h;
Stage #2: with sodium tetrahydroborate in methanol;ethanol at 0 - 20;
Stage #3: with water in methanol;ethanol at 0;
Steps:
60.a
a) Preparation of 3-methyl-2-(methylaminomethyl)indole; Methylamine (0.34 niL, 8.4 mmol, 33% in ethanol) was added to a solution of 3- methylindole-2-carboxaldehyde (447 mg, 2.8 mmol) in methanol (10 mL) and stirred for 5 hours. Sodium borohydride (104 mg, 2.8 mmol) was slowly added at 00C. The resultant mixture was warmed to room temperature and stirred overnight. Water (2 mL) was added slowly at 0 0C and the mixture was evaporated to a paste. The paste was partitioned
References:
WO2007/53131,2007,A2 Location in patent:Page/Page column 166-167
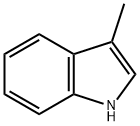
83-34-1
512 suppliers
$12.00/10g

50-00-0
896 suppliers
$10.00/25g

74-89-5
0 suppliers
$13.44/25ML

894852-67-6
14 suppliers
$369.00/500mg