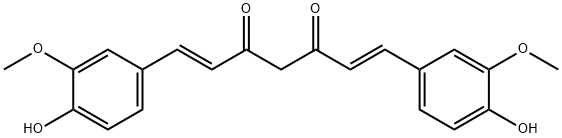
Curcumin synthesis
- Product Name:Curcumin
- CAS Number:458-37-7
- Molecular formula:C21H20O6
- Molecular Weight:368.38
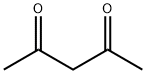
121-33-5
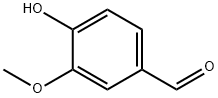
123-54-6

458-37-7
GENERAL METHOD: Acetylacetone (10 mmol) was dissolved in ethyl acetate (30 mL) and boronic anhydride (5.0 mmol) was added. Subsequently, vanillin (20 mmol) or 4-hydroxybenzaldehyde (20 mmol) and tributyl borate (40 mmol) were added to the solution. The reaction mixture was stirred at 50 °C for 10 min. Next, a solution of n-butylamine (5.0 mmol) in ethyl acetate (5 mL) was slowly added dropwise at 15 °C. After completion of the dropwise addition, the reaction mixture was warmed to 80 °C and stirring was continued for 4 hours. Upon completion of the reaction, the reaction was quenched by the addition of 1N hydrochloric acid (30 mL) and stirring was continued for 40 minutes. The organic layer was separated, washed with water and dried over anhydrous sodium sulfate. The solvent was removed by evaporation under reduced pressure and the residue was recrystallized from methanol to give the target products (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione (curcuminoids 1a and 1b) as a yellow solid.

121-33-5
1183 suppliers
$9.00/1g

123-54-6
582 suppliers
$10.00/25ml

458-37-7
973 suppliers
$5.00/250mg
Yield:458-37-7 85%
Reaction Conditions:
Stage #1: vanillin;acetylacetonewith diboron trioxide;boric acid tributyl ester in ethyl acetate at 50; for 0.166667 h;
Stage #2: with N-butylamine in ethyl acetate at 50 - 80; for 4.25 h;
Steps:
3.2. General Method for the Synthesis of CCM (1a) and BCM (1b)
General procedure: Acetylacetone (10 mmol) was added to a solution of boric anhydride (5.0 mmol) in ethyl acetate (30 mL), followed by the addition of vanillin (20 mmol) or 4-hydroxybenzaldehyde (20 mmol) and tributyl borate (40 mmol). The reaction mixture was stirred at 50 °C for 10 min. Subsequently, n-butylamine (5.0 mmol) in ethyl acetate (5 mL) was added dropwise over 15 min at 50 °C and additionally stirred for 4 hours at 80 °C. Hydrochloric acid (1 N, 30 mL) was added, and the mixture was stirred for another 40 min. The organic layers were washed with water, dried with Na2SO4. The solvent was evaporated, and the residue was recrystallized from methanol to give the corresponding curcuminoids (1a and 1b) as yellow solids.
References:
Cao, Ya-Kun;Li, Hui-Jing;Song, Zhi-Fang;Li, Yang;Huai, Qi-Yong [Molecules,2014,vol. 19,# 10,p. 16349 - 16372]

121-33-5
1183 suppliers
$9.00/1g

123-54-6
582 suppliers
$10.00/25ml

458-37-7
973 suppliers
$5.00/250mg
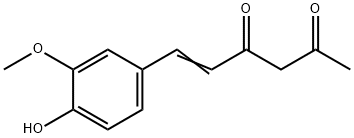
1924-25-0
2 suppliers
inquiry

121-33-5
1183 suppliers
$9.00/1g

458-37-7
973 suppliers
$5.00/250mg