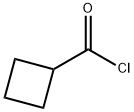
Cyclobutanecarbonyl chloride synthesis
- Product Name:Cyclobutanecarbonyl chloride
- CAS Number:5006-22-4
- Molecular formula:C5H7ClO
- Molecular Weight:118.56
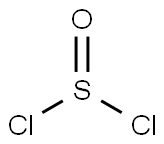
7719-09-7
416 suppliers
$15.00/10g
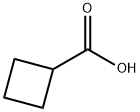
3721-95-7
423 suppliers
$7.00/5g

5006-22-4
199 suppliers
$12.00/1g
Yield:-
Reaction Conditions:
with N,N-dimethyl-formamide in tetrahydrofuran at 20; for 2 h;
Steps:
5.46 N-[5-({2-[(Cyclopropylcarbonyl)amino][1,2,4]triazolo[1,5-a]pyridin-6-yl}oxy)-2-methylphenyl]-2,5-dimethyl-1,3-thiazole-4-carboxamide (29a)
General procedure: To a solution of 2,5-dimethyl-1,3-thiazole-4-carboxylic acid (107mg, 0.681mmol) in THF (5mL) was added thionyl chloride (118μL, 1.36mmol) and two drops of DMF. The mixture was stirred at room temperature for 2h and concentrated under reduced pressure. The residue was dissolved in DMA (6mL), and 27b (200mg, 0.619mmol) was added to the solution at 0°C. The mixture was stirred at room temperature for 2h. The mixture was diluted with saturated aqueous sodium hydrogen carbonate and extracted with AcOEt. The extract was washed with saturated aqueous sodium hydrogen carbonate, brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residual solid was recrystallized from EtOH to give 29a (248mg, 87%) as white crystals: mp 239-240°C; 1H NMR (DMSO-d6) δ 0.78-0.86 (4H, m), 2.04 (1H, br s), 2.23-2.28 (3H, m), 2.65 (3H, s), 2.70 (3H, s), 6.82 (1H, dd, J=8.3, 2.7Hz), 7.27 (1H, d, J=8.7Hz), 7.50 (1H, dd, J=9.5, 2.3Hz), 7.66 (1H, d, J=2.7Hz), 7.69-7.75 (1H, m), 8.82 (1H, dd, J=2.3, 0.8Hz), 9.66 (1H, s), 11.02 (1H, s); Anal. Calcd for C23H22N6O3S: C, 59.73; H, 4.79; N, 18.17. Found: C, 59.48; H, 4.80; N, 18.00.
References:
Oguro, Yuya;Cary, Douglas R.;Miyamoto, Naoki;Tawada, Michiko;Iwata, Hidehisa;Miki, Hiroshi;Hori, Akira;Imamura, Shinichi [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 15,p. 4714 - 4729]

3721-95-7
423 suppliers
$7.00/5g

5006-22-4
199 suppliers
$12.00/1g
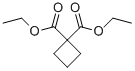
3779-29-1
270 suppliers
$19.00/10g

5006-22-4
199 suppliers
$12.00/1g
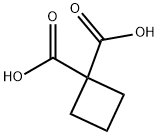
5445-51-2
465 suppliers
$8.00/5g

5006-22-4
199 suppliers
$12.00/1g
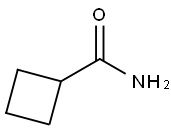
1503-98-6
85 suppliers
$29.00/100mg

5006-22-4
199 suppliers
$12.00/1g