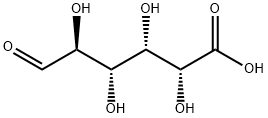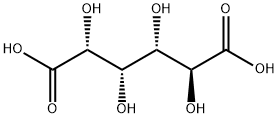
D-Glucaric acid synthesis
- Product Name:D-Glucaric acid
- CAS Number:87-73-0
- Molecular formula:C6H10O8
- Molecular Weight:210.14
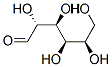
2280-44-6
12 suppliers
inquiry

87-73-0
138 suppliers
inquiry
Yield:87-73-0 60%
Reaction Conditions:
with 2.44 wt.% platinum and 2.38 wt.% aurum on carbon;oxygen in water monomer at 90; under 4635.46 Torr; for 5 h;Reagent/catalyst;
Steps:
2.B.ii Oxidation of Glucose to Glucaric Acid (Protocol 2)
Suitably concentrated aqueous solutions of K2Pt(OH)6 and CsAu02 were added together to carbon black powders by incipient wetness impregnation and agitated to impregnate the supports. The samples were dried in an oven at 40°C overnight, and reduced at 250°C under a forming gas (5% H2 and 95% N2) atmosphere for 3 hours with 5 °C/min temperature ramp rate. The final catalysts were washed with deionized water and finally dried at 40°C to produce catalysts with a composition of 2.44 wt.% Pt and 2.38 wt.% Au. By using other carbon black supports, Au and Pt precursors, and adjusting amount of Au and Pt in solution, different catalysts with various Au and Pt loadings on a variety of commercial carbon black powders or particles from extrudates were prepared in a similar manner. These catalysts were tested for glucose oxidation using the following testing protocol. Catalyst (10 mg) was weighed into a glass vial insert followed by addition of an aqueous glucose solution (250 μ of 0.55 M). The glass vial insert was loaded into a reactor and the reactor was closed. The atmosphere in the reactor was replaced with oxygen and pressurized to 618 kPa (75 psig) at room temperature. Reactor was heated to 90°C and maintained at the respective temperature for 5 hours while vials were shaken. After that, shaking was stopped and reactor was cooled to 40°C. Pressure in the reactor was then slowly released. The glass vial insert was removed from the reactor and centrifuged. The solution was diluted with deionized water and analyzed by ion chromatography to determine the yield of glucaric acid and the selectivity as defined herein. Results are presented in Table 4.
References:
WO2017/75391,2017,A1 Location in patent:Paragraph 0327-0328
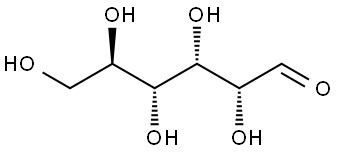
50-99-7
1091 suppliers
$6.00/25g
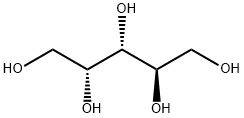
488-82-4
249 suppliers
$7.00/100mg
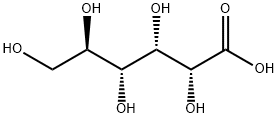
526-95-4
423 suppliers
$5.00/5g

87-73-0
138 suppliers
inquiry

50-99-7
1091 suppliers
$6.00/25g

87-73-0
138 suppliers
inquiry