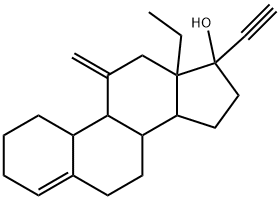
Desogestrel synthesis
- Product Name:Desogestrel
- CAS Number:54024-22-5
- Molecular formula:C22H30O
- Molecular Weight:310.47
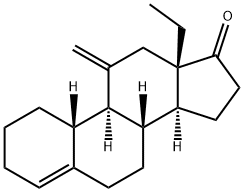
54024-21-4
101 suppliers
$130.00/10mg
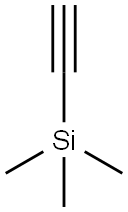
1066-54-2
468 suppliers
$9.00/10g

54024-22-5
322 suppliers
$29.00/5mg
Yield:54024-22-5 89%
Reaction Conditions:
Stage #1:trimethylsilylacetylene with n-hexyllithium in tetrahydrofuran;hexane at -5; for 0.5 h;
Stage #2:11-methylene-18a-homo-estr-4-en-17-one in tetrahydrofuran;hexane at 0 - 5; for 1 h;
Steps:
17 Example 17. Synthesis of Desoqestrel (ethynylation reaction)
To a solution of hexillithium (3.0 g 2.3 M in hexane) in hexane (5 mL) cooled at - 5°C, a solution of trimethylsilyl acetylene (1 .32 g) in 6.8 mL of a mixture of THF/hexane1/7 was slowly added. The reaction mixture was stirred at -5°C for 30 mm, then asolution of compound 14 (1 .0 g) in 8 mL of hexane was added and the mixture stirred for 1 h at 0/5°C. Aqueous NaCI solution (8.5 mL) was added and the phases were separated. The organic phase was mixed with 5 mL of methanol, followed by addition of 1.5 mL of aqueous NaOH 30% and stirred for 4 h further. 10 mL of an aqueous solution of 3% Acetic acid was added. The phases were separated and the organicphase was washed with water (5.0 mL). The solvent was evaporated under reduced pressure and the residue dissolved in 1 mL of MeOH. The solvent was evaporated under reduced pressure and the residue dissolved in 2 mL of Hexane. The crude obtained was dissolved in 4 mL of hexane by heating at 60°C. The solution was cooled slowly in an ice bath and the resulting solid filtered, washed with 1 mL of cold hexaneand dried at 40°C under vacuum, to yield 0.89 g of Desogestrel (89%).
References:
BIONICE, S.L.U;SANDOVAL RODRÍGUEZ, Celso Miguel;HERRÁIZ SIERRA, Ignacio;MESSINA, Ivano;IGLESIAS RETUERTO, Jesús Miguel WO2017/149091, 2017, A1 Location in patent:Page/Page column 36; 37

54024-21-4
101 suppliers
$130.00/10mg

74-86-2
78 suppliers
inquiry

54024-22-5
322 suppliers
$29.00/5mg

1216963-74-4
0 suppliers
inquiry

54024-21-4
101 suppliers
$130.00/10mg

54024-22-5
322 suppliers
$29.00/5mg

54024-21-4
101 suppliers
$130.00/10mg
110-54-3
825 suppliers
$20.00/100ml

54024-22-5
322 suppliers
$29.00/5mg
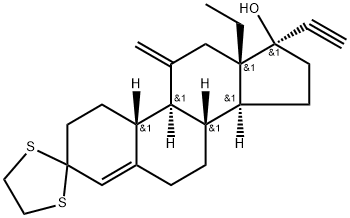
116930-33-7
1 suppliers
inquiry

54024-22-5
322 suppliers
$29.00/5mg