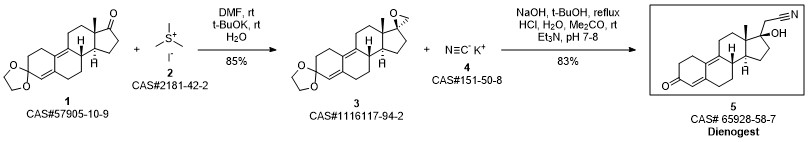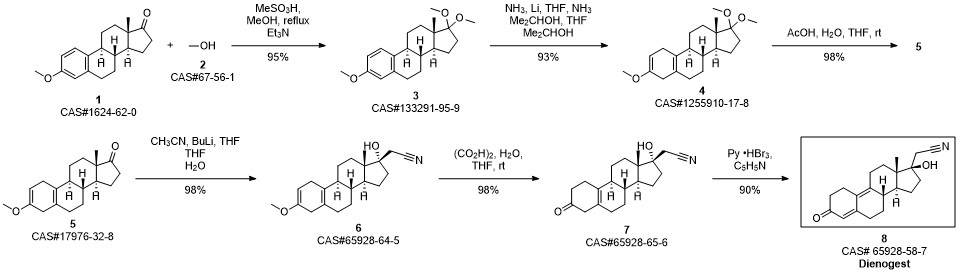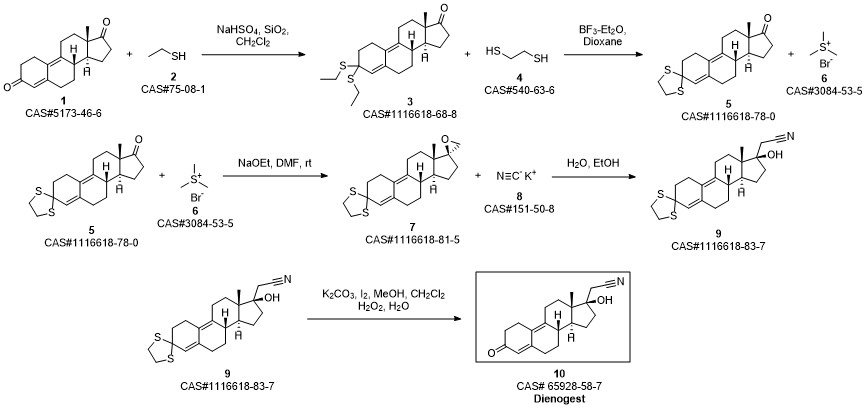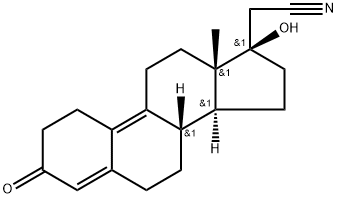
Dienogestrel synthesis
- Product Name:Dienogestrel
- CAS Number:65928-58-7
- Molecular formula:C20H25NO2
- Molecular Weight:311.43
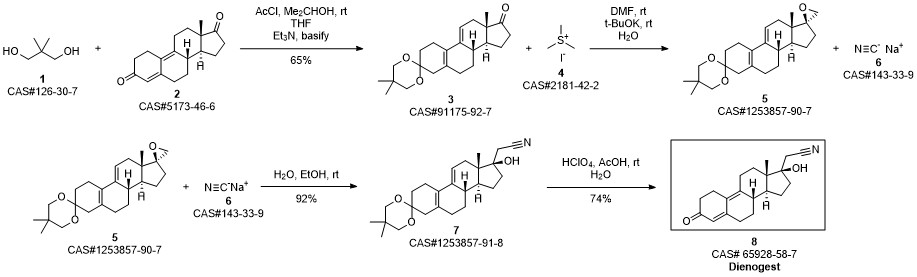
Liang, Yong; Chen, Bo; Wang, Shaojie. Synthesis of dienogest. Shenyang Yaoke Daxue Xuebao. Volume 28. Issue 10. Pages 788-790. Journal. (2011).
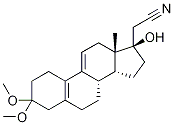
102193-41-9
37 suppliers
inquiry

65928-58-7
387 suppliers
$40.00/100 mg
Yield:65928-58-7 99.9 %Chromat.
Reaction Conditions:
with perchloric acid in water;acetonitrile at 20 - 30; for 1 h;Reagent/catalyst;Solvent;
Steps:
3 Preparation of Crude Dienogest
The compound (100 g) of example-1 in acetonitrile (600 mL) was cooled to 0-5° C. and 70% perchloric acid (150 mL) was added and stirred for one hour at 20-30° C.
After completion of reaction, water (1500 mL) was added and solid was filtered to obtain crude product. (HPLC data: Dienogest-99.55%, diene impurity-0.125%). Purification of Crude Dienogest a) Wet solid from example-2 was charcoalised in dimethylformamide (400 mL) at 45-50° C. for one hour, filtered, washed with dimethylformamide (100 ml) and to the combined filtrate water (200 ml) was added. Reaction mass stirred for 2 hours at 0-5° C. Solid was filtered and dried under reduced pressure at 40-45° C. to give 66 g crude dienogest. (HPLC data: Dienogest - 99.79%, Diene impurity-0.02%).b) Dienogest (25 g) from example 3(a) was taken in dimethylformamide (125 mL) and heated to 40-50° C., filtered, washed with dimethylformamide (25 ml) and to the combined filtrate water (37.5 ml) was added to precipitate the product, cooled to 0 to 5° C. and stirred for 2 hours. Solid obtained was filtered, washed with water and dried under vacuum at 40-45° C. to give 23 g of Dienogest.(HPLC data: Dienogest - 99.9%, Diene impurity - 0.02%).
References:
LUPIN LIMITED;Tambe, Suhas Ganpat;Kirange, Bhushan Bhanudas;Singh, Gurvinder Pal;Ray, Purna Chandra US2013/41166, 2013, A1 Location in patent:Paragraph 0040
![(17α)-3,3-[1,2-Ethanediylbis(oxy)]-17-hydroxy-19-norpregna-5(10),9(11)-diene-21-nitrile](/CAS/GIF/190662-30-7.gif)
190662-30-7
30 suppliers
inquiry

65928-58-7
387 suppliers
$40.00/100 mg
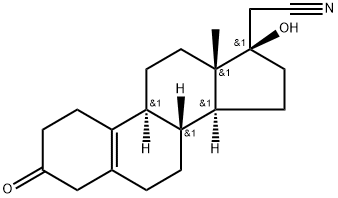
65928-65-6
19 suppliers
inquiry

65928-58-7
387 suppliers
$40.00/100 mg

102193-41-9
37 suppliers
inquiry
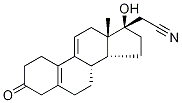
106111-42-6
35 suppliers
inquiry

65928-58-7
387 suppliers
$40.00/100 mg
6218-29-7
122 suppliers
$13.00/200mg

75-05-8
1044 suppliers
$10.00/10g

65928-58-7
387 suppliers
$40.00/100 mg