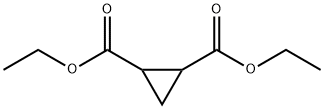
DIETHYL 1,2-CYCLOPROPANEDICARBOXYLATE synthesis
- Product Name:DIETHYL 1,2-CYCLOPROPANEDICARBOXYLATE
- CAS Number:20561-09-5
- Molecular formula:C9H14O4
- Molecular Weight:186.21
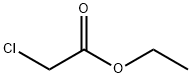
105-39-5
426 suppliers
$10.00/5g
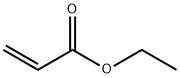
140-88-5
559 suppliers
$12.00/100ml

20561-09-5
50 suppliers
$76.00/100mg
Yield:-
Reaction Conditions:
with sodium hydride in ethanol;toluene;mineral oil at 30 - 50; for 6 h;
Steps:
Diethyl Cyclopropane-1,2-dicarboxylate (1)
The mixture of ethyl acrylate (150 g, 1.5 mol) and ethylchloroacetate (184 g, 1.5 mol) was slowly added to a mixtureof 72g(1.8mol) NaH (60% dispersion in mineral oil) and 300mL anhydrous toluene with mechanic stirring. After about20 mL of the mixture of ethyl acrylate and ethyl chloroacetatewas added, 2 mL ethanol was added with drops to inspirethe reaction. The reaction produced bubbles and thetemperature was controlled between 40~50°Cby an icewaterbath. After the addition was completed for 4 hours, thereaction was kept at 30°C for another 2 hours. The dark yellowreaction mixture was treated with about 10 mL ofmethanol (to decompose any excessive sodium hydride) followedby addition of 300 mL water to dissolve the inorganicsalts. The toluene layer was separated and the aqueous portionwas extracted with toluene (150mL2). The combinedorganic layer was washed with brine (250 mL2) and driedover MgSO4. After removal of solvent in vacuum, 1 wasobtained by distillation (9mmHg, 106~112°C) as colorlesstransparent oil liquid (163g, HPLC: 84.52%, cis-and transisomers,yield: 58%).
References:
Wang, Fan;Xu, Xiao-Ying;Wang, Fei-Ying;Peng, Lin;Zhang, Yong;Wang, Liang-Liang;Wang, Li-Xin [Letters in Organic Chemistry,2015,vol. 12,# 10,p. 741 - 744]
623-73-4
151 suppliers
$26.69/5g

140-88-5
559 suppliers
$12.00/100ml

20561-09-5
50 suppliers
$76.00/100mg
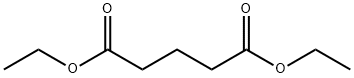
818-38-2
207 suppliers
$34.00/25g

20561-09-5
50 suppliers
$76.00/100mg

623-73-4
151 suppliers
$26.69/5g

140-88-5
559 suppliers
$12.00/100ml
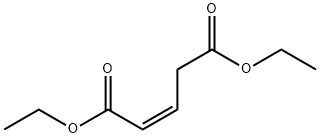
73192-75-3
0 suppliers
inquiry
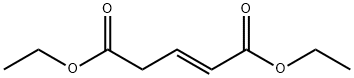
73178-43-5
1 suppliers
inquiry

20561-09-5
50 suppliers
$76.00/100mg