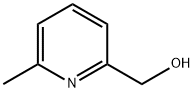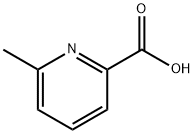
6-Methyl-2-pyridinecarboxylic acid synthesis
- Product Name:6-Methyl-2-pyridinecarboxylic acid
- CAS Number:934-60-1
- Molecular formula:C7H7NO2
- Molecular Weight:137.14
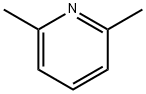
108-48-5

934-60-1
Step 1: 2,6-dimethylpyridine (10.71 g, 0.1 mol) was dissolved in 500 mL of deionized water, stirred and heated to 60 °C. Subsequently, potassium permanganate (KMnO4, 23.7 g, 0.15 mol) was added in ten portions, with an interval of 30 min between each addition. The temperature was strictly controlled at about 60°C during the reaction, which lasted for 5 h and then stopped. Immediately after completion of the reaction, the insoluble impurities were removed by filtration. The filtrate was adjusted to pH=5 and then extracted with ethyl acetate. The organic phase was collected, concentrated under reduced pressure and finally recrystallized from ethanol to obtain 6-methyl-2-pyridinecarboxylic acid (10.3 g, 75% yield). In this step, the effects of different reaction conditions (e.g., equivalents of KMnO4 of 1, 1.5 and 2, aqueous potassium permanganate solution of different pH, temperature and molar ratio) on the reaction were investigated by orthogonal tests, and the optimal reaction conditions were determined to be neutral aqueous potassium permanganate solution with a dosage of 1.5 equivalents of KMnO4.

108-48-5
489 suppliers
$6.09/190ul

934-60-1
268 suppliers
$8.00/1g
Yield: 75%
Reaction Conditions:
with potassium permanganate in water-d2 at 20 - 60; for 5 h;
Steps:
1.1
Step 1: compound 1 (10.71g, 0 . 1mol) dissolved in 500 ml deionized water, stirred and heated up to 60 °C. Then the 23.7g (0.15mol) the KMnO4 solid-in into the above-mentioned solution for ten times, the time interval between the two times for 30 min. Strict control of temperature in the 60 °C left and right, the reaction at room temperature to 5h. After the reaction is ended immediately used to remove insoluble impurity. PH=5 level to the acidity, then using the ethyl acetate extraction, collecting the organic phase, decompression turns on lathe does, recrystallized with ethanol to obtain compound 2 (10.3g, 75%).In this step of trying to adopt different reaction condition such as KMnO4equivalent are respectively 1,1.5,2, potassium permanganate adopt different PH, temperature, molar compared to passing through orthogonal experiment to obtain the optimal conditions for neutral potassium permanganate aqueous solution, KMnO4to 1.5 equivalent.
References:
Shanghai Institute Of Technology;Yao, Zhiyi;Zhang, Xiaopan;Zong, Haoqiang;Wang, Dongsheng CN105294552, 2016, A Location in patent:Paragraph 0047; 0048; 0049; 0050
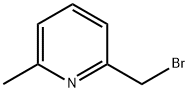
68470-59-7
124 suppliers
$20.00/250mg

934-60-1
268 suppliers
$8.00/1g
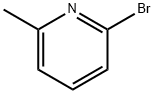
5315-25-3
465 suppliers
$6.00/5g

934-60-1
268 suppliers
$8.00/1g
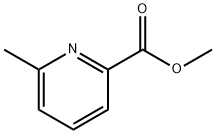
13602-11-4
171 suppliers
$9.00/1g

934-60-1
268 suppliers
$8.00/1g