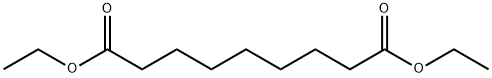
DIETHYL AZELATE synthesis
- Product Name:DIETHYL AZELATE
- CAS Number:624-17-9
- Molecular formula:C13H24O4
- Molecular Weight:244.33
Yield:624-17-9 88%
Reaction Conditions:
with hydrogenchlorideHeating / reflux;
Steps:
1
A mixture of azelaic acid (20 g) and 200 proof USP anhydrous ethanol (200 ml) was placed into a 350 ml gas washing bottle. Into this solution anhydrous EPO
References:
WO2006/74379,2006,A2 Location in patent:Page/Page column 18-19

334-43-0
9 suppliers
inquiry
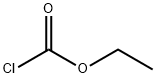
541-41-3
0 suppliers
$21.00/5g

332-97-8
0 suppliers
inquiry

624-17-9
107 suppliers
$40.00/5g

4549-31-9
199 suppliers
$12.00/1g

624-17-9
107 suppliers
$40.00/5g
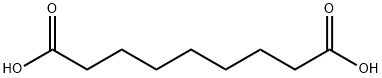
123-99-9
778 suppliers
$14.00/5g

624-17-9
107 suppliers
$40.00/5g

123-99-9
778 suppliers
$14.00/5g
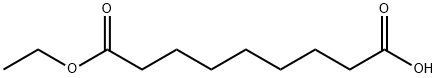
1593-55-1
30 suppliers
$130.00/100mg

624-17-9
107 suppliers
$40.00/5g
