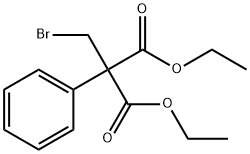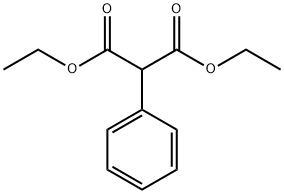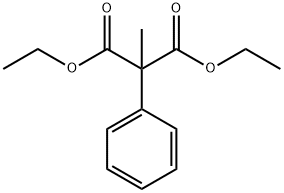
DIETHYL METHYLPHENYLMALONATE synthesis
- Product Name:DIETHYL METHYLPHENYLMALONATE
- CAS Number:34009-61-5
- Molecular formula:C14H18O4
- Molecular Weight:250.29
Yield:-
Reaction Conditions:
with heptamethyl cobyrinate perchlorate in N,N-dimethyl-formamide;Electrolysis;Irradiation;Inert atmosphere;
Steps:
General procedure: The controlled-potential electrolysis of 3 (0.10M) was carried out in the presence of 1 (9.3×10-4M) in DMF containing n-Bu4NBF4 (0.50M) in a cylindrical three-electrode cell with a single sheet of a microporous polypropylene membrane which was divided into two internal compartments under a nitrogen atmosphere. Platinum mesh working counter electrodes and an Ag-AgCl reference electrode were used in this system. The applied potential between the working and reference electrodes during the electrolysis was maintained constant by a Hokuto Denko HA-305 potentiostat/galvanostat, and the reaction was monitored by a Hokuto Denko HF-201 coulomb/ampere-hour meter. The light irradiation was carried out by a 300W tungsten lamp. After the electrolysis, DMF was removed by evaporation under reduced pressure and 30mL of CHCl3 was added to the residue. The chloroform layer was washed with water (3×40mL) to completely remove the DMF then dried over Na2SO4. The filtrate was then concentrated to dryness. The residue was passed through a silica gel short column eluting with CHCl3 to remove the n-Bu4NPF6 and 2, and the products were then analyzed by GC. The GC data were obtained using a Shimadzu GC-4C or GC-9APF apparatus
References:
Abe, Masaaki;Hisaeda, Yoshio;Pan, Ling;Shimakoshi, Hisashi;Tahara, Keishiro;Yamaguchi, Ryoko [Journal of Inorganic Biochemistry,2017,vol. 177,p. 438 - 443]
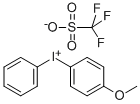
115298-63-0
5 suppliers
inquiry
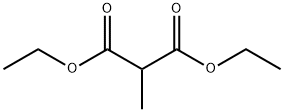
609-08-5
403 suppliers
$5.00/10g
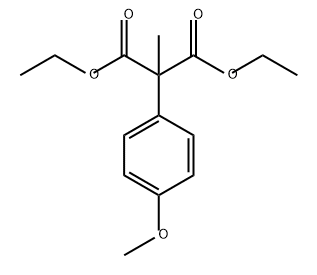
74610-81-4
0 suppliers
inquiry

34009-61-5
15 suppliers
inquiry

108-86-1
524 suppliers
$10.00/5g

105-53-3
791 suppliers
$5.00/25g

74-88-4
357 suppliers
$15.00/10g

34009-61-5
15 suppliers
inquiry