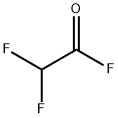
DIFLUOROACETYL FLUORIDE synthesis
- Product Name:DIFLUOROACETYL FLUORIDE
- CAS Number:2925-22-6
- Molecular formula:C2HF3O
- Molecular Weight:98.02
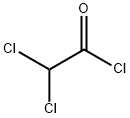
79-36-7
296 suppliers
$15.00/10g

2925-22-6
33 suppliers
inquiry
Yield:2925-22-6 99%
Reaction Conditions:
with potassium fluoride;C30H22Cl4CrN4O2 at 80; for 2 h;Reagent/catalyst;
Steps:
2.1; 2.2; 2.3
Add 147g (1mol) of dichloroacetyl chloride, 180g of potassium fluoride and 6.9g (0.01mol) of chromium complex catalyst into the pressure-resistant reactor, connect the valve and gas conduit, and raise the temperature to 80. After the reaction for 2 hours, open the valve, the target product difluoroacetyl fluoride flows out from the conduit in the form of gas, and a small amount of the product is introduced into the container containing dichloromethane by the conduit to form a difluoroacetyl fluoride solution in dichloromethane, which is subjected to gas phase The result of chromatographic analysis is consistent with the standard sample of difluoroacetyl fluoride. Then, while continuing to maintain the reaction at 80°C, the generated difluoroacetyl fluoride is introduced into a container for storage or directly introduced into other reactions until the system no longer emits gas. The reaction formula is as follows:
References:
CN111995516,2020,A Location in patent:Paragraph 0041; 0045-0046; 0058; 0062-0063; 0066; 0070-0071
512-51-6
180 suppliers
$17.00/5g

2925-22-6
33 suppliers
inquiry
425-88-7
145 suppliers
$15.00/10g

2925-22-6
33 suppliers
inquiry

425-88-7
145 suppliers
$15.00/10g
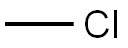
74-87-3
222 suppliers
$18.50/48622
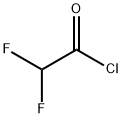
381-72-6
52 suppliers
inquiry

2925-22-6
33 suppliers
inquiry
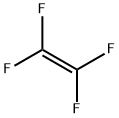
116-14-3
54 suppliers
inquiry

2925-22-6
33 suppliers
inquiry