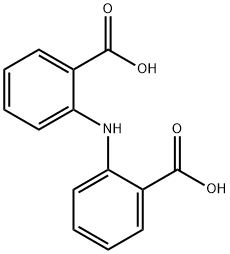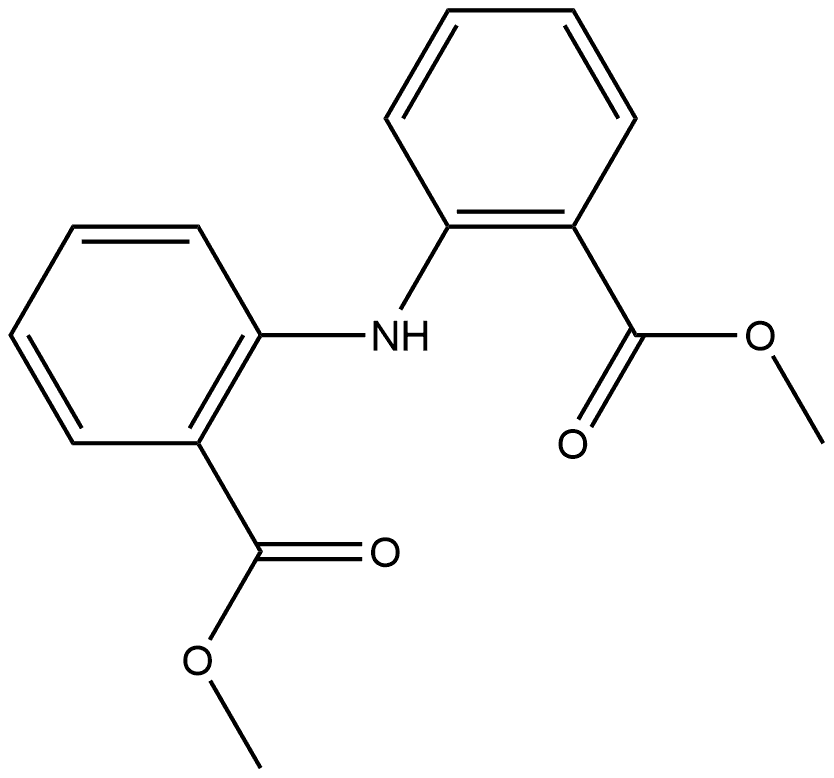
dimethyl 2,2'-azanediyldibenzoate synthesis
- Product Name:dimethyl 2,2'-azanediyldibenzoate
- CAS Number:34069-89-1
- Molecular formula:C16H15NO4
- Molecular Weight:285.29
Yield:34069-89-1 98%
Reaction Conditions:
with thionyl chloride for 12.33 h;Cooling with ice;Reflux;
Steps:
6 2.6 Synthesis of 2-[2-(methoxycarbonyl)phenylamino]benzoic acid methyl ester (4b)
This compound was synthesized by a similar procedure to that reported for the chloro-derivative of this compound with some modifications [37]. To a stirred solution of 3b (6.20g, 24.12mmol) in MeOH (124mL) in an ice bath, thionyl chloride (6.1mL) was added dropwise. This solution was stirred for 20min at room temperature and then refluxed for 12h. The dark solution was then concentrated to half volume and put aside. The resulting pale green crystals were collected and dried. Yield: 6.4g (98%); m.p. 92°C; FT-IR (KBr, cm-1): ν(N-H), 3309 sh; ν(C-Haliphatic), 2985-2940; ν(C=O), 1700. 1H NMR (300MHz, DMSO-d6): δ=10.72(s, 1H, NH), 7.92-6.95 (m, 8H, Ar-H), 3.85 (s, 6H, CH3).
References:
Yousefi, Maryam;Sedaghat, Tahereh;Simpson, Jim;Motamedi, Hossein;Dayer, Mohammad Reza [Polyhedron,2018,vol. 155,p. 153 - 162]
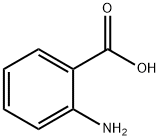
118-92-3
0 suppliers
$23.00/250g

34069-89-1
4 suppliers
inquiry
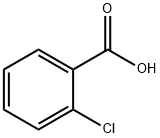
118-91-2
672 suppliers
$14.00/25g

34069-89-1
4 suppliers
inquiry