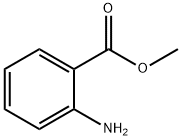
Methyl anthranilate
- CAS No.
- 134-20-3
- Chemical Name:
- Methyl anthranilate
- Synonyms
- METHYL 2-AMINOBENZOATE;Methyl antranilate;NEROLI;Nevoli oil;Methyl anthranylate;o-Carbomethoxyaniline;ANTHRANILIC ACID METHYL ESTER;2-amino-benzoicacimethylester;2-AMINOBENZOIC ACID METHYL ESTER;FEMA 2682
- CBNumber:
- CB0722306
- Molecular Formula:
- C8H9NO2
- Molecular Weight:
- 151.16
- MDL Number:
- MFCD00007710
- MOL File:
- 134-20-3.mol
- MSDS File:
- SDS
| Melting point | 24 °C (lit.) |
|---|---|
| Boiling point | 256 °C (lit.) |
| Density | 1.168 g/mL at 25 °C (lit.) |
| vapor pressure | 1 mm Hg ( 20 °C) |
| refractive index |
n |
| FEMA | 2682 | METHYL ANTHRANILATE |
| Flash point | 220 °F |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | alcohol: freely soluble(lit.) |
| form | Liquid |
| pka | pK1:2.23(+1) (25°C) |
| color | Clear yellow-brown |
| Odor | grape odor |
| PH | 7.5-8 (H2O, 20℃)Aqueous solution |
| explosive limit | 1.4-7.8%(V) |
| Viscosity | 7.14mm2/s |
| Odor Type | fruity |
| Water Solubility | slightly soluble |
| Sensitive | Air Sensitive |
| Merck | 14,6020 |
| JECFA Number | 1534 |
| BRN | 606965 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | VAMXMNNIEUEQDV-UHFFFAOYSA-N |
| LogP | 1.88 at 20℃ |
| Substances Added to Food (formerly EAFUS) | METHYL ANTHRANILATE |
| FDA 21 CFR | 182.60; 582.60 |
| CAS DataBase Reference | 134-20-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 981I0C1E5W |
| NIST Chemistry Reference | Benzoic acid, 2-amino-, methyl ester(134-20-3) |
| Pesticides Freedom of Information Act (FOIA) | Methyl anthranilate |
| EPA Substance Registry System | Methyl anthranilate (134-20-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36-37/39 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | CB3325000 | |||||||||
| F | 21 | |||||||||
| Autoignition Temperature | 986 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29224995 | |||||||||
| Toxicity | LD50 orally in rats, mice: 2910, 3900 mg/kg, P. M. Jenner et al., Food Cosmet. Toxicol. 2, 327 (1964) | |||||||||
| NFPA 704 |
|
Methyl anthranilate price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W268224 | Methyl Anthranilate natural, ≥98%, FG | 134-20-3 | 25g | $108 | 2024-03-01 | Buy |
| Sigma-Aldrich | W268224 | Methyl Anthranilate natural, ≥98%, FG | 134-20-3 | 100g | $323 | 2024-03-01 | Buy |
| Sigma-Aldrich | W268224 | Methyl Anthranilate natural, ≥98%, FG | 134-20-3 | 1kg | $1830 | 2024-03-01 | Buy |
| Sigma-Aldrich | W268208 | Methyl Anthranilate ≥98%, FCC, FG | 134-20-3 | sample-k | $54.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | W268208 | Methyl Anthranilate ≥98%, FCC, FG | 134-20-3 | 1kg | $70.9 | 2024-03-01 | Buy |
Methyl anthranilate Chemical Properties,Uses,Production
Description
Methyl anthranilate, also known as MA, methyl 2-amino benzoate or carbo methoxy aniline, is an ester of anthranilic acid. Its chemical formula is C8H9NO2.
Chemical Properties
Methyl anthranilate has a characteristic orange-flower odor and a slightly bitter, pungent taste. May be prepared by heating anthranilic acid and methyl alcohol in the presence of sulfuric acid and subsequent distillation.
Chemical Properties
Methyl Anthranilate occurs in a large
number of blossom essential oils (e.g., neroli, ylang-ylang, and jasmine oils),
grapes, and citrus oils. It occurs as white crystals (mp 24–25°C), or a yellowish
liquid, that show blue fluorescence and have an orange blossom odor. Methyl
anthranilate is prepared by esterification of anthranilic acid with methanol or by
reaction of isatoic anhydride with methanol.
It is used in a large number of blossom fragrances. However, its use in perfumes
for soaps and cosmetics is limited because it causes discoloration. It is used in
flavor compositions (e.g., in grape and citrus flavors).
Occurrence
Methyl anthranilate naturally occurs in the Concord grapes and other Vitis labrusca grapes or hybrids thereof, and in bergamot, black locust, champaca , gardenia, jasmine, lemon, mandarin, neroli, oranges, rue oil, strawberry, tuberose, wisteria, galangal and ylang ylang. It is also a primary component of the essential apple flavor, along with ethyl acetate and ethyl butyrate.It is also secreted by the musk glands of foxes and dogs, and lends a "sickly sweetness" to the smell of rotting flesh.
Uses
Methyl anthranilate acts as a bird repellent. It is food-grade and can be used to protect corn, sunflowers, rice, fruit, and golf courses. Dimethyl anthranilate (DMA) has a similar effect. It is also used for the flavor of grape Kool Aid. It is used for flavoring of candy, soft drinks (e.g. grape soda), gums, and drugs.
Methyl anthranilate both as a component of various natural essential oils and as a synthesised aroma-chemical is used extensively in modern perfumery . It is also used to produce Schiff's Bases with aldehydes, many of which are also used in perfumery. In a perfumery context the most common Schiff's Base is known as aurantiol - produced by combining methyl anthranilate and hydroxyl citronellal.
Definition
ChEBI: Methyl anthranilate is a benzoate ester that is the methyl ester of anthranilic acid. It has a role as a metabolite and a flavouring agent. It derives from an anthranilic acid.
Preparation
By heating anthranilic acid and methyl alcohol in the presence of sulfuric acid and subsequent distillation.
Synthesis Reference(s)
Tetrahedron Letters, 33, p. 3599, 1992 DOI: 10.1016/S0040-4039(00)92512-7
General Description
Clear colorless to tan liquid with an odor of grapes. Has light blue fluorescence.
Air & Water Reactions
Methyl anthranilate is sensitive to air and light. Slightly water soluble .
Reactivity Profile
An amine and ester. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Fire Hazard
Methyl anthranilate is combustible.
Toxicology
Methyl anthranilate is a colorless liquid that has a sweet, fruity, grape-like flavor. It is found in the essential oils of orange, lemon, and jasmine and has been widely used to create imitation Concord grape flavor. Table 10.8 shows the acute toxicity of methyl anthranilate. Methyl anthranilate promotes some allergic reactions on human skin, which has led to it being prohibited for use in cosmetic products.
Safety Profile
Moderately toxic by ingestion. Experimental reproductive effects. A skin irritant. See also ESTERS. Combustible liquid. When heated to decomposition it emits toxic fumes of NOx.
Safety
Methyl anthranilate is a plant-based compound with a long history of use as a flavor additive for foods and beverages, and as an aromatic used extensively in perfumery. As such, the US Department of Agriculture (USDA) and the Food and Drug Administration (FDA) have approved MA as "generally recognized as safe".
Metabolism
It is probable that this ester is hydrolysed and the anthranilate is excreted mostly as oaminobenzoyl glucuronide (Charconnet-Harding, Dalgliesh & Neuberger, 1953).
Solubility in organics
Methyl anthranilate is soluble in ethanol and propylene glycol. It is insoluble in paraffin oil.
Toxicity evaluation
Even though MA is palatable to humans, it is an irritant to birds. The bird-repellent properties of MA and related compounds were discovered in the late 1950s (25). The mode of action is via the trigeminal nerve. Thus, all avian species tested so far perceive MA as an irritant, not as a taste repellent per se.
Methyl anthranilate Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Changzhou AniKare Pharmatech Co., Ltd. | +86-0519-8359-8696 +8618018249389 | sales@anikare.com | China | 8382 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7845 | 58 |
| Hunan aslsen technology co.,ltd | +8619313031929 | aslsd@aslsen.com | China | 128 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 | sales03@chemcn.cn | China | 951 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 2259 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
Related articles
- Methyl anthranilate: production and applications
- Methyl anthranilate, a natural metabolite with a characteristic grape-like odor, can now be directly produced via microbial fe....
- Jun 16,2023
- What is Methyl anthranilate?
- Methyl anthranilate, also known as MA, methyl 2-aminobenzoate, or carbomethoxyaniline, is an ester of anthranilic acid manufac....
- Jan 2,2020
View Lastest Price from Methyl anthranilate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Methyl anthranilate
134-20-3
|
US $2.00 / KG | 1KG | 99% | 1000mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-04-26 | Methyl anthranilate
134-20-3
|
US $30.00 / kg | 1kg | 98% | 2000kg | hebei hongtan Biotechnology Co., Ltd | |
 |
2024-04-22 | Methyl anthranilate
134-20-3
|
US $10.00 / kg | 1kg | 99% | 1000 tons | Hunan aslsen technology co.,ltd |
-

- Methyl anthranilate
134-20-3
- US $2.00 / KG
- 99%
- Jinan Finer Chemical Co., Ltd
-

- Methyl anthranilate
134-20-3
- US $30.00 / kg
- 98%
- hebei hongtan Biotechnology Co., Ltd
-

- Methyl anthranilate
134-20-3
- US $10.00 / kg
- 99%
- Hunan aslsen technology co.,ltd