
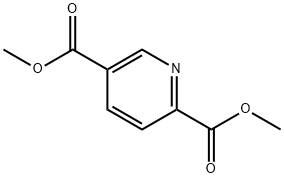
Dimethyl 2,4-pyridinedicarboxylate synthesis
- Product Name:Dimethyl 2,4-pyridinedicarboxylate
- CAS Number:25658-36-0
- Molecular formula:C9H9NO4
- Molecular Weight:195.17

67-56-1
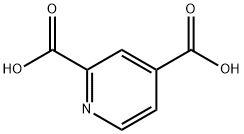
499-80-9

25658-36-0
Phosphorus pentachloride (PCl5, 39 g, 0.186 mol, 3 eq.) and 2,4-pyridinedicarboxylic acid (11.5 g, 6.2 x 10^-2 mol) were added to a 500 mL round bottom flask. The mixture was stirred at room temperature for 45 minutes and the mixture was observed to transform into a green liquid. Subsequently, the reaction mixture was diluted with dichloromethane (80 mL) and methanol (20 mL) was added slowly. The reaction mixture was poured into water (300 mL) containing sodium bicarbonate (NaHCO3, 60 g), the aqueous phase was extracted with dichloromethane (4 x 50 mL) and the combined organic phases were washed with 1 M sodium carbonate (Na2CO3, 2 x 50 mL). The crude product was purified by fast column chromatography (eluent: ethyl acetate/cyclohexane, 75:25). A solid product of 11.71 g was obtained in 96% yield. The product was stored at room temperature.1H NMR (200 MHz, CDCl3): δ 4.03 (s, 3H, -OCH3), 4.09 (s, 3H, -OCH3), 8.07 (dd, J = 6 Hz, 1H, H-3), 8.68 (s, 1H, H-5), 8.91 (d, 1H, H-2).13C NMR (75 MHz. CDCl3): δ 53.28 (-OCH3), 124.49 (C-5), 126.29 (C-3), 138.64 (C-4), 149.06 (C-6), 150.92 (C-2), 162.45 (C=O), 164.96 (C=O).

67-56-1
790 suppliers
$9.00/25ml

499-80-9
293 suppliers
$8.00/1g

25658-36-0
64 suppliers
$45.00/50mg
Yield:25658-36-0 98%
Reaction Conditions:
with thionyl chloride for 5 h;Reflux;
Steps:
3.3 5.1.2.4 Preparation of dimethyl [2,2'-bipyridine]-4,4'-dicarboxylate (17)
General procedure: Thionyl chloride (300μL, 4.08mmol) was added to a suspension of 16 (400mg, 1.62mmol) in MeOH (30mL) in a dropwise fashion.
The mixture was heated at reflux temperature overnight.
Then, the solvent was removed under reduced pressure and the residue was partitioned between CH2Cl2 and water.
The organic layer was washed with saturated aqueous NaHCO3 solution and dried over Na2SO4.
Filtration, evaporation in vacuo, and recrystallization in AcOEt gave 17 (400mg, 91%)
References:
Miyake, Yuka;Itoh, Yukihiro;Hatanaka, Atsushi;Suzuma, Yoshinori;Suzuki, Miki;Kodama, Hidehiko;Arai, Yoshinobu;Suzuki, Takayoshi [Bioorganic and Medicinal Chemistry,2019,vol. 27,# 6,p. 1119 - 1129]

499-80-9
293 suppliers
$8.00/1g
149-73-5
550 suppliers
$12.00/5g

25658-36-0
64 suppliers
$45.00/50mg

67-56-1
790 suppliers
$9.00/25ml

499-80-9
293 suppliers
$8.00/1g

25658-36-0
64 suppliers
$45.00/50mg
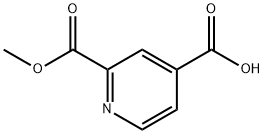
24195-10-6
58 suppliers
$58.00/100mg
2459-07-6
235 suppliers
$5.00/1g

124-38-9
134 suppliers
$214.00/14L

74-88-4
357 suppliers
$15.00/10g
881-86-7
177 suppliers
$28.00/1g

25658-36-0
64 suppliers
$45.00/50mg

499-80-9
293 suppliers
$8.00/1g

25658-36-0
64 suppliers
$45.00/50mg