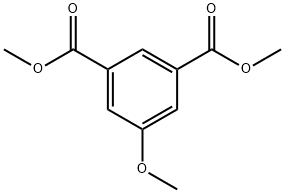
DIMETHYL 5-METHOXYISOPHTHALATE synthesis
- Product Name:DIMETHYL 5-METHOXYISOPHTHALATE
- CAS Number:20319-44-2
- Molecular formula:C11H12O5
- Molecular Weight:224.21
13036-02-7
182 suppliers
$6.00/5g

74-88-4
357 suppliers
$15.00/10g

20319-44-2
70 suppliers
$45.00/50mg
Yield:20319-44-2 100%
Reaction Conditions:
with potassium carbonate in acetone at 20;
Steps:
1 Example 1; 3-Cyano-5-methoxybenzoic acid
A solution of [DIMETHYL-5-HYDROXYISOPHTHALATE] (6 g, 28. 6 mmol) and potassium carbonate (9 g, 65.4 mmol) in acetone (120 [ML)] was prepared. To this, methyl iodide (4 [ML,] 63.7 mmol) was added and the reaction was left stirring overnight at room temperature. The reaction mixture was filtered and then concentrated. The residue was dissolved in ethyl acetate and washed with water and brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to yield 6.4 g (quantitative) of dimethyl-5-methoxy-isophthalate as an [OFF-WHITE SOLID. LH NMR (CDC13), 6 (PPM)] : 8. 28 (s, 1H), 7.75 (s, 2H), 3.95 (s, 6H), 3.90 (s, 3H). A suspension of dimethyl-5-methoxy-isophthalate (6.4 g, 28.5 mmol) in methanol (143 mL) was treated with 1 N sodium hydroxide (25.6 mL, 25.6 mmol). The reaction was left stirring overnight at room temperature. After the solution was concentrated, the residue was dissolved in water and transferred to a separatory funnel. The aqueous layer was washed with dichloromethane (3 times) and then acidified with 1 N [HC1] to pH 2. Ethyl acetate was used to extract the precipitate, which was then washed with brine and dried over anhydrous sodium sulfate. After removal of solvent in vacuo, 4.5 g (75%) of 5- methoxyisophthalic acid monomethyl ester was isolated as a white [SOLID. 1H] NMR (DMSO), 8 (ppm): 8.17 (m, 1H), 7.60 (m, 2H), 3.80 (s, 3H), 3.76 (s, 3H). A suspension of 5-methoxyisophthalic acid monomethyl ester [(4. 5 MG,] 21.3 mmol) in thionyl chloride (25 mL) was heated at reflux for 3 h. The excess thionyl chloride was then removed in vacuo and the intermediate acid chloride dissolved in dichloromethane (20 mL). After cooling to [0 °C] the solution was treated with 0.5 M ammonia in 1,4-dioxane (102 mL) and then allowed to warm to room temperature. After 1.5 h of stirring the solvent was removed in vacuo and the residue was triturated with water. The precipitate was collected, washed with water and dried in vacuo to afford 4.0 g (90 %) of 5-methoxy- isophthalamic acid methyl ester as an [OFF-WHITE SOLID. 1H] NMR [(CDC13),] [8] (ppm): 8.11 (s, 1H), 7.68 (m, 2H), 3.95 (s, 3H), 3.91 (s, 3H). A suspension of 5-methoxy-isophthalamic acid methyl ester (4.0 g, 19.1 mmol) in a dichloromethane (80 mL) at [0 °C] was treated with pyridine (6.3 mL, 77.0 mmol) and then trifluoroacetic anhydride drop-wise (6.5 mL, 46 mmol). The reaction was stirred at 0 [°C] for 20 min. and then stirred overnight at room temperature. The reaction mixture was washed with water, [1.] 0 N [HC1] and brine, dried over anhydrous sodium sulfate, filtered and concentrated to afford 3.6 g (98%) [OF 3-CYANO-5-METHOXY-BENZOIC] acid methyl ester as a white solid. A solution of 3-cyano-5-methoxy-benzoic acid methyl ester (3.4 g, 18. 7 mmol) in THF (45 mL) was treated with 0.5 N lithium hydroxide (45 mL, 22.4 mmol). The reaction was stirred at [75°C] for 2 h and then the solvent was removed in vacuo. The residue was dissolved in a small amount of water and then acidified (pH 2) by the addition of 1 N [HC1.] Ethyl acetate was used to extract the precipitate, which was then washed with brine and dried over anhydrous sodium sulfate. After removal of solvent in vacuo, 2.5 g (77%) of 3- [CYANO-5-METHOXYBENZOIC] acid was isolated as a white [SOLID. 1H] NMR (DMSO), [8] (ppm): 7. 86 (s, [1H),] 7.71 (m, 2H), 3. 87 (s, 3H).
References:
WO2004/14902,2004,A2 Location in patent:Page 37-38
618-83-7
312 suppliers
$9.00/10g

74-88-4
357 suppliers
$15.00/10g

20319-44-2
70 suppliers
$45.00/50mg

618-83-7
312 suppliers
$9.00/10g

77-78-1
319 suppliers
$22.00/25g

20319-44-2
70 suppliers
$45.00/50mg
46331-50-4
128 suppliers
$11.00/250mg

20319-44-2
70 suppliers
$45.00/50mg

618-83-7
312 suppliers
$9.00/10g

20319-44-2
70 suppliers
$45.00/50mg