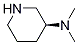
DiMethyl-(S)-piperidin-3-yl-aMine synthesis
- Product Name:DiMethyl-(S)-piperidin-3-yl-aMine
- CAS Number:1061873-15-1
- Molecular formula:C7H16N2
- Molecular Weight:128.2153
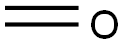
50-00-0
896 suppliers
$10.00/25g
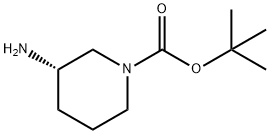
625471-18-3
295 suppliers
$14.14/1gm:
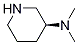
1061873-15-1
7 suppliers
inquiry
Yield:1061873-15-1 55%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in dichloromethane;water at 0 - 20; for 16 h;
Steps:
23 Synthesis of (S)-3-dimethylaminopiperidine
An aqueous solution of formalin (36-38 wt %, 2.10 g, 25.2 mmol), sodium triacetoxyborohydride (2.13 g, 10.0 mmol), and acetic acid (0.0300 g, 0.500 mmol) were added to a solution of (S)-3-amino-1-tert-butoxycarbonylpiperidine (1.00 g, 4.99 mmol) in dichloromethane (10.0 mL) at 0° C., and the resulting mixture was stirred at room temperature for 16 hours. The reaction liquid was cooled to 0° C. A saturated aqueous solution of sodium hydrogencarbonate was added to the reaction liquid, and the resulting mixture was extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated under reduced pressure. The residue was dissolved in hydrochloric acid (1.0 N), and the resulting mixture was extracted with ethyl acetate. A 48% aqueous solution of sodium hydroxide was added to the aqueous layer for basification, and then the resulting mixture was extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated under reduced pressure. The residue was dissolved in methanol (25.0 mL), and concentrated hydrochloric acid (5.0 mL) was added, and then the resulting mixture was stirred at 40° C. for 12 hours. The reaction liquid was concentrated and exsiccated, and then the residue was dissolved in distilled water. A 48% aqueous solution of sodium hydroxide was added for basification, and then the resulting mixture was extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated under reduced pressure. (S)-3-Dimethylaminopiperidine (0.351 g, 2.74 mmol, 55%) was obtained as a colorless oil.
References:
TW2016/2093,2016,A Location in patent:Paragraph 0321

1217636-04-8
4 suppliers
inquiry
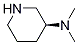
1061873-15-1
7 suppliers
inquiry