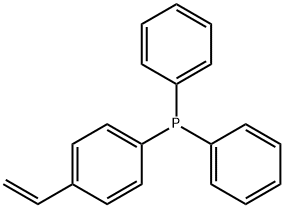
DIPHENYL(P-VINYLPHENYL)PHOSPHINE synthesis
- Product Name:DIPHENYL(P-VINYLPHENYL)PHOSPHINE
- CAS Number:40538-11-2
- Molecular formula:C20H17P
- Molecular Weight:288.32
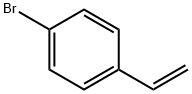
2039-82-9
303 suppliers
$10.00/1g
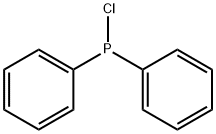
1079-66-9
450 suppliers
$5.00/5g

40538-11-2
65 suppliers
$30.30/1g
Yield: 94%
Reaction Conditions:
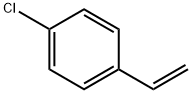
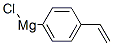
Stage #1:1-bromo-4-ethenyl-benzene with iodine;magnesium;hydroquinone in tetrahydrofuran at 20; for 2 h;Sealed tube;Inert atmosphere;Grignard Reaction;
Stage #2:chloro-diphenylphosphine in tetrahydrofuran at 30; for 4 h;Cooling with ice;Temperature;
Steps:
1.1 First step, synthesis of compound 2a ((4-vinylphenyl)diphenylphosphine)
Magnesium powder was separately washed with hydrochloric acid and acetone and spare dried. THF is refluxed with metal Na to benzophenone till it changes into dark purple and spare evaporated. A freshly prepared 1.5 g Mg powder (1.1 eq) was added to the sealed reaction device with drop and reflux function , and replaced with N2 three times. Under the protection of N2, a small amount of I2 particles, 5 mg of HQ (p-diphenyl acid) and refined THF (about 10 ml) were added in order, and the dried compound 1 (4-bromostyrene) (10 g) was transferred to a dropping funnel. Diluted with purified THF (10 ml). The reaction was initiated by heating. After the initiation, a solution of 4-bromostyrene in THF was slowly added dropwise, and the dropping rate was controlled so that the solution temperature did not exceed 30°C. After the addition was completed, the mixture was stirred at room temperature for 2 hours to obtain a gray mixture. After the Grignard reagent is prepared, the reaction apparatus was transferred to an ice-water bath, cooled and stirred for 15 minutes, and a solution of chlorodiphenylphosphine(12 g) in THF was slowly added dropwise to the Grignard reagent, dropping temperature controlled, dropping completed, reaction was continuously stirred at 30 ° C 4h. The reaction mixture was slowly added to a 10% aqueous solution of ice-hydrochloric acid, extracted with ethyl acetate, dried, suction-filtered, and the solvent was evaporated under reduced pressure to give a crude white solid. Recrystallization to give a white solid. Yield: 94%.
References:
Dalian University of Technology;Jiang Wenfeng;Fan Lingxia;Yuan Xiao;Zhang Tianbao;Wang Huilong CN107573379, 2018, A Location in patent:Paragraph 0029; 0030; 0040
47182-95-6
5 suppliers
inquiry

40538-11-2
65 suppliers
$30.30/1g