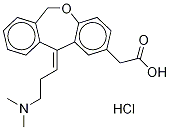
(E)-Olopatadine Hydrochloride synthesis
- Product Name:(E)-Olopatadine Hydrochloride
- CAS Number:949141-22-4
- Molecular formula:C21H24ClNO3
- Molecular Weight:373.8732
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![[3-(Dimethylamino)propyl]triphenylphosphonium bromide hydrobromide](/CAS/GIF/27710-82-3.gif)
27710-82-3
202 suppliers
inquiry

949141-22-4
38 suppliers
$215.00/1mg
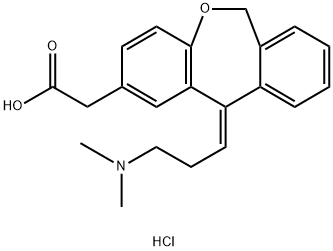
140462-76-6
497 suppliers
$5.00/10mg
Yield: 99% , 0.5%
Reaction Conditions:
Stage #1:anhydrous 3-(dimethylamino)propyltriphenylphosphonium bromide hydrobromide with sodium hydride in tetrahydrofuran for 3 h;Heating / reflux;Wittig Reaction;
Stage #2:(11-oxo-6,11-dihydro-dibenzo[b,e]oxepin-2-yl)-acetic acid benzyl ester in tetrahydrofuran at 0 - 30; for 3 h;
Stage #3: with hydrogenchloride;water in di-isopropyl ether; pH=2
Steps:
Charged 238 gms [3 - (Dimethylamino) propylamine] triphenyl phosphonium bromide hydrobromide in 1400 ml tetrahydrofuran under nitrogen atmosphere. Slowly charged 130.5 gms sodium hydride and raised the temperature to reflux to maintain for 3.0 hrs. The
References:
INDOCO REMEDIES LIMITED WO2009/81417, 2009, A2 Location in patent:Page/Page column 11-12
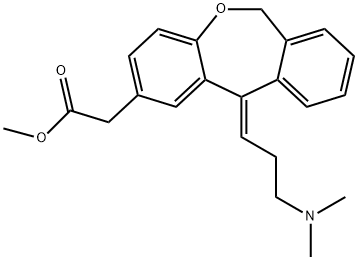
![Dibenz[b,e]oxepin-2-acetic acid, 11-[3-(dimethylamino)propylidene]-6,11-dihydro-](/CAS/20210111/GIF/113805-63-3.gif)
113805-63-3
2 suppliers
inquiry

949141-22-4
38 suppliers
$215.00/1mg

140462-76-6
497 suppliers
$5.00/10mg
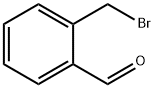
113806-02-3
5 suppliers
inquiry

949141-22-4
38 suppliers
$215.00/1mg
60633-91-2
41 suppliers
$125.00/50mg

949141-22-4
38 suppliers
$215.00/1mg
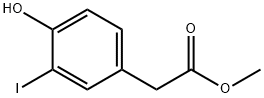
352469-17-1
16 suppliers
inquiry

949141-22-4
38 suppliers
$215.00/1mg