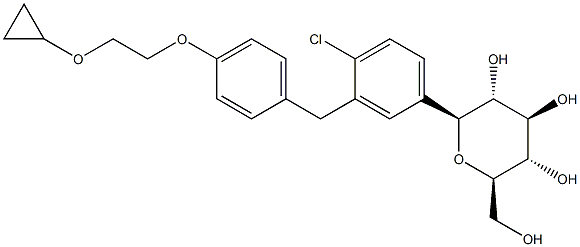
Bexagliflozin synthesis
- Product Name:Bexagliflozin
- CAS Number:1118567-05-7
- Molecular formula:C24H29ClO7
- Molecular Weight:464.94
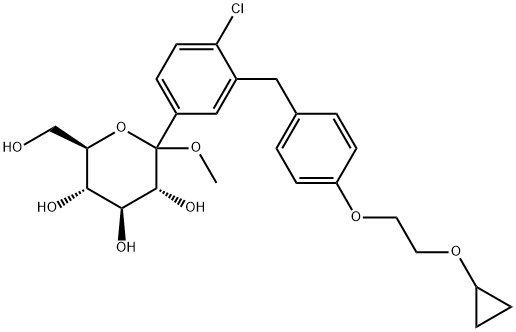
1459754-30-3

1118567-05-7
Example 5: Preparation of (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-(2-cyclopropoxyethoxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol [0236] This embodiment describes the preparation of (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-(2-cyclopropoxyethoxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol by reduction of end-isomers OMe and/or OH. Procedure. 1. Add (3R,4S,5S,6R)-2-(4-chloro-3-(4-(2-cyclopropoxyethoxy)benzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol crude (2.7 kg), dichloromethane (5.4 kg), and acetonitrile (3.2 kg) to a 30L glass reactor under nitrogen protection with magnetic stirred until complete dissolution. 2. In another vessel, boron trifluoride ethyl ether complex (2.34 kg) was added dropwise to a mixture of dichloromethane (5.4 kg) and acetonitrile (3.2 kg) of triethylsilane (2.55 kg) at -21 to -15 °C, maintaining a nitrogen atmosphere. 3. The feedstock solution prepared in step 1 was slowly added to the cold solution in step 2, and the addition rate was controlled to maintain the reaction temperature between -20 and -25°C (about 3 hours). After addition, the reaction continues to be stirred at -22 to -25°C for 4 hours. 4. Upon completion of the reaction, the reaction was quenched by the slow addition of 7.4% w/w aqueous sodium bicarbonate (18.3 kg), controlling the internal temperature to no more than -10°C. The reaction was then quenched by the addition of solid sodium bicarbonate (18.3 kg). Subsequently, solid sodium bicarbonate (1.35 kg) was added to adjust the pH to 7.5. 5. The solvent was removed by depressurized concentration (temperature < 40 °C) and the residue was partitioned with ethyl acetate (18 kg) and water (9.2 kg). The organic phase was separated and the aqueous phase was extracted with ethyl acetate (2 x 9 kg). The organic layers were combined and washed with saturated brine (2 x 9 kg). 6. The organic phase was concentrated to near dryness (temperature < 40 °C) under pressure, added anhydrous ethanol (9 kg) and concentrated again to give the crude product (2.5 kg, yield 90%, HPLC purity 90.8%, method HPLC-0001) as a foamy solid.

1459754-30-3
1 suppliers
inquiry

1118567-05-7
133 suppliers
$25.00/1mg
Yield: 90%
Reaction Conditions:
with triethylsilane;boron trifluoride diethyl etherate in dichloromethane;acetonitrile at -25 - -20; for 4 h;Inert atmosphere;Large scale;Temperature;Concentration;
Steps:
5 Example 5 Preparation of ((2S,3R,4R,5S,6R)-2-(4-Chloro-3-(4-(2-Cyclopropoxyethoxy)Benzyl)Phenyl)-6-(Hydroxymethyl)Tetrahydro-2H-Pyran-3,4,5-triol
Example 5 Preparation of ((2S,3R,4R,5S,6R)-2-(4-Chloro-3-(4-(2-Cyclopropoxyethoxy)Benzyl)Phenyl)-6-(Hydroxymethyl)Tetrahydro-2H-Pyran-3,4,5-triol [0236] This example describes preparation of (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-(2-cyclopropoxyethoxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol by reduction of the anomeric OMe and/or OH. (3R,4S,5S,6R)-2-(4-Chloro-3-(4-(2-Cyclopropoxyethoxy)Benzyl)Phenyl)-6-(Hydroxymethyl)-2-Methoxytetrahydro-2H-Pyran-3,4,5-Triol Solution [0237] A 30 L glass reactor equipped with a thermometer was charged with crude (3R,4S,5S,6R)-2-(4-chloro-3-(4-(2-cyclopropoxyethoxy)benzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol (2.7 kg), DCM (5.4 kg) and acetonitrile (3.2 kg), and the mixture was magnetically stirred until all the solids dissolved under nitrogen sparging. [0238] Triethylsilane Solution: [0239] BF3.Et2O (2.34 kg) was added to a cold (-21 to -15° C.) solution of triethysilane (2.55 kg) dichloromethane (5.4 kg) and acetonitrile (3.2 kg) under nitrogen. [0240] The (3R,4S,5S,6R)-2-(4-chloro-3-(4-(2-cyclopropoxyethoxy)benzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol solution was added to the cold triethylsilane solution at such a rate to maintain the temperature between -20 and -25° C. (3 h). [0241] The reaction mixture was stirred for another 4 h at -22 to -25° C. and then quenched by addition of an aqueous solution of sodium bicarbonate (7.4% w/w, 18.3 kg) while keeping the internal temperature below -10° C. Solid sodium bicarbonate (1.35 kg) was added to adjust the pH to 7.5. The solvents were removed under reduced pressure (temperature below 40° C.). The residue was partitioned between ethyl acetate (18 kg) and water (9.2 kg). The layers were separated and the aqueous layer was extracted with ethyl acetate (2×9 kg). The combined organic layers were washed with brine (2×9 kg) and the solvents were removed under reduced pressure at the temperature below 40° C. until the condensation almost stop Anhydrous ethanol (9 kg) was added and concentrated to give the crude product of the title compound (2.5 kg, 90% yield, 90.8% HPLC purity, HPLC-0001) as foamy solid.
References:
Theracos, Inc.;Xu, Baihua;Lv, Binhua;Xu, Ge;Seed, Brian;Roberge, Jacques Y. US2013/267694, 2013, A1 Location in patent:Paragraph 0236; 0237; 0238; 0239; 0240; 0241
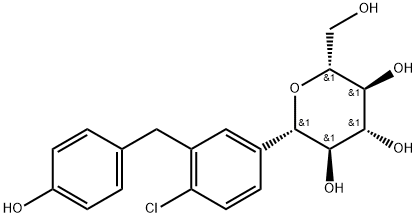
![α-D-Glucopyranoside, methyl 1-C-[4-chloro-3-[[4-[2-(cyclopropyloxy)ethoxy]phenyl]methyl]phenyl]-](/CAS/20211123/GIF/1118567-46-6.gif)
1118567-46-6
1 suppliers
inquiry

1118567-05-7
133 suppliers
$25.00/1mg
864070-37-1
95 suppliers
inquiry
862728-59-4
65 suppliers
$41.00/250mg

1118567-05-7
133 suppliers
$25.00/1mg
18742-02-4
304 suppliers
$6.00/5g

1118567-05-7
133 suppliers
$25.00/1mg
21739-92-4
679 suppliers
$8.00/10g

1118567-05-7
133 suppliers
$25.00/1mg