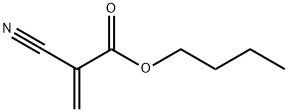
enbucrilate synthesis
- Product Name:enbucrilate
- CAS Number:6606-65-1
- Molecular formula:C8H11NO2
- Molecular Weight:153.18

50-00-0
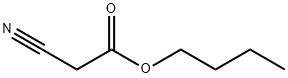
5459-58-5

6606-65-1
The general procedure for the synthesis of embutane from formaldehyde and n-butyl cyanoacetate is as follows: 400 g of n-butyl cyanoacetate and an aqueous solution of paraformaldehyde or formaldehyde were added to a reactor, followed by 5 g of triphenylphosphine and 10 g of 1,5,7-triazacyclo[5.5.0]dec-5-ene. The mixture was stirred thoroughly and the reactor pressure was maintained below 30 mmHg using a control device. The reactor temperature was increased from room temperature to 60-65°C at a ramp rate of 1-1.5°C/min for 30 minutes for the condensation reaction. Next, the temperature was raised to 70-75°C at the same rate for 15 minutes for the reaction, and then the temperature was continued to 80-85°C and held for 15 minutes. The molar ratio of n-butyl cyanoacetate to formaldehyde was controlled at 1.05:1. After completion of the reaction, hot air was blown at 50 °C for 1 h to dry the reaction mixture, which was followed by treatment at 70 °C and 3 mmHg for 10 min to remove water completely. After dehydration, 5 g of phosphorus pentoxide and 5 g of hydroquinone were added and a vacuum of 3 mmHg was maintained while the reactor temperature was increased from room temperature to 120-130 °C at a rate of 1-1.5 °C/min for 15 min for the condensation reaction. The temperature was continued to be raised to 150-170 °C at the same rate and maintained for 15 min, and finally raised to 190-220 °C for 30 min to obtain crude α-cyanoacrylic acid n-butyl ester. The crude product was distilled by rapidly increasing the temperature, applying vacuum and collecting the fractions to obtain a distillation product with 95.3% yield and 98.6% GC purity.
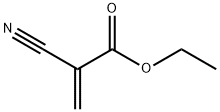
7085-85-0
199 suppliers
$19.00/25g

71-36-3
1092 suppliers
$14.00/25mL

6606-65-1
133 suppliers
$60.00/25 mg
Yield:6606-65-1 440 g
Reaction Conditions:
with phosphoric acid;sulfuric acid;toluene-4-sulfonic acid;acetic acid;chloroacetic acid at 100 - 115;Reagent/catalyst;Temperature;
Steps:
2; 3; 4; 5; 7; 8; 9; 10 Preparation method of n-butyl cyanoacrylate:
500 g of the α-cyanoacrylate ethyl ester monomer prepared in Example 1 was placed in a 1000 ml flask, and a mixture of 2% glacial acetic acid and anhydrous phosphoric acid (the molar ratio of glacial acetic acid to anhydrous phosphoric acid was 0.7:1) was added. 0.35% of hydroquinone and 0.45% of a mixture of concentrated sulfuric acid and p-toluenesulfonic acid (the molar ratio of concentrated sulfuric acid to p-toluenesulfonic acid is 1:1), adding 444 g of n-butanol, stirring and heating, controlling the pot temperature of 100 -115 ° C, collect the ethanol fraction of the top of 76-82 ° C; increase the temperature of the kettle to 120-140 ° C, normal pressure and pump decompression (-0.1MPa) to collect n-butanol, oil pump decompression (200-800Pa) Fractions with >98% content were collected until the temperature at the top of the column decreased significantly. 440 g of n-butyl cyanoacrylate having a content of >99% was obtained.
References:
Hebei Chengxin Group Co., Ltd.;Wang Hong;Li Chengguo;Zhang Chaochun;Fan Chunyan;Sun Jiehao;Liu Shaojun;Dong Jin CN109810021, 2019, A Location in patent:Paragraph 0061; 0062; 0063; 0064; 0065; 0066; 0067-0085

50-00-0
897 suppliers
$10.00/25g

5459-58-5
205 suppliers
$12.00/25g

6606-65-1
133 suppliers
$60.00/25 mg

5459-58-5
205 suppliers
$12.00/25g

6606-65-1
133 suppliers
$60.00/25 mg

2568-90-3
69 suppliers
$15.00/25mL

5459-58-5
205 suppliers
$12.00/25g

6606-65-1
133 suppliers
$60.00/25 mg
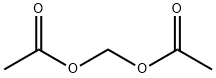
628-51-3
70 suppliers
$12.00/250mg

5459-58-5
205 suppliers
$12.00/25g

6606-65-1
133 suppliers
$60.00/25 mg