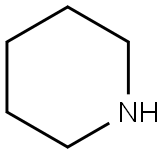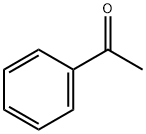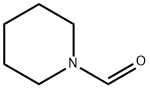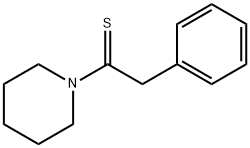
Ethanethione,2-phenyl-1-(1-piperidinyl)- synthesis
- Product Name:Ethanethione,2-phenyl-1-(1-piperidinyl)-
- CAS Number:24815-46-1
- Molecular formula:C13H17NS
- Molecular Weight:219.35
Yield:24815-46-1 85%
Reaction Conditions:
with sulfur in neat (no solvent) at 100; for 0.583333 h;Green chemistry;
Steps:
General procedure for the Willgerodt-Kindler reaction
General procedure: To a mixture of ketone (2 mmol), sulfur (2.4 mmol), and secondary amines (2.4 mmol) sulfated polyborate(10 wt%) was added. The mixture was heated at 100 °C in an oil bath. The reaction was monitored by thin-layer chromatography. After completion of the reaction, the mixture was cooled to room temperature, diluted with ethyl acetate (3 9 5 mL) and the catalyst was removed by filtration. The combined organic layers were washed with water, dried over sodium sulfate and evaporated under reduced pressure to afford crude products, which were purified by column chromatography using petroleum ether and ethyl acetate solvent system. The products obtained were known compounds and were identified by 1H NMR spectroscopy. The spectral data were compared with those in the literature.
References:
Rekunge, Deelip S.;Khatri, Chetan K.;Chaturbhuj, Ganesh U. [Monatshefte fur Chemie,2017,vol. 148,# 12,p. 2091 - 2095]
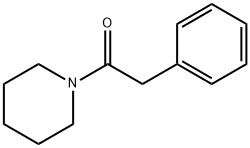
3626-62-8
1 suppliers
inquiry

24815-46-1
8 suppliers
inquiry
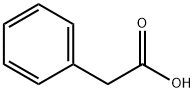
103-82-2
0 suppliers
$22.60/100g

24815-46-1
8 suppliers
inquiry
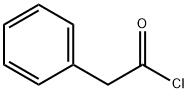
103-80-0
322 suppliers
$13.00/25g

24815-46-1
8 suppliers
inquiry