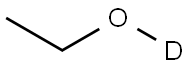
ETHANOL-D synthesis
- Product Name:ETHANOL-D
- CAS Number:925-93-9
- Molecular formula:C2H5DO
- Molecular Weight:47.07

141-78-6
685 suppliers
$22.37/250ml
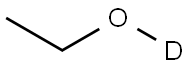
925-93-9
93 suppliers
$58.50/25ml
Yield:-
Reaction Conditions:
with [D]-sodium hydroxide;water-d2 at 75;Inert atmosphere;
Steps:
5-6
Add 60mL (0.608mol, purity 99%) of ethyl acetate and 60mL of NaOD solution with a concentration of 11mol/L (containing 0.66mol of NaOD, the abundance of NaOD is 99.9%) into a flat-bottomed flask with a capacity of 500mL, and then add 111g (5.54 mol, abundance 99.9%) of heavy water, filled with nitrogen, stirred and refluxed at 75°C until ethyl acetate was completely hydrolyzed, the stirring speed was 550r/min, then the reaction product was transferred to an atmospheric distillation device, and replaced with nitrogen in the device The air, and then heated to collect the distillate at 165 ° C,Obtain the alcohol-water mixture of 164.4g, then the alcohol-water mixture is heated and collected the distillate under 85 , obtains the monodeuterated ethanol of 88.8g (the deuterated rate is 68.9%, and purity is 27.8%, and heavy water content is 72.2% , yield by ethanol is 51.9%, deuterium atom utilization rate is 64.7%), and the remaining liquid of distillation is reclaimed, obtains the recovery heavy water of 69.1g.
References:
CN115572211,2023,A Location in patent:Paragraph 0056-0065

64-17-5
825 suppliers
$10.00/50g
925-93-9
93 suppliers
$58.50/25ml
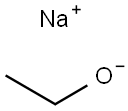
141-52-6
498 suppliers
$10.00/5g
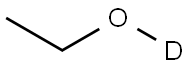
925-93-9
93 suppliers
$58.50/25ml
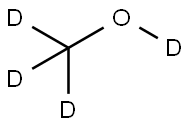
811-98-3
222 suppliers
$18.69/1g

141-78-6
685 suppliers
$22.37/250ml
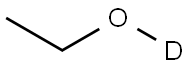
925-93-9
93 suppliers
$58.50/25ml
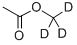
24704-57-2
13 suppliers
$502.62/5MG