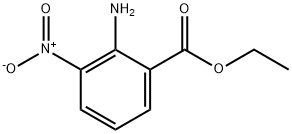
ethyl 2-amino-3-nitro-benzoate synthesis
- Product Name:ethyl 2-amino-3-nitro-benzoate
- CAS Number:61063-11-4
- Molecular formula:C9H10N2O4
- Molecular Weight:210.19
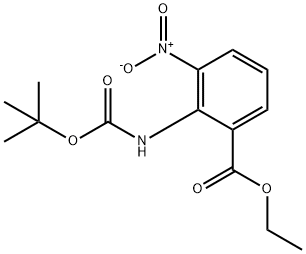
136285-65-9
135 suppliers
inquiry

61063-11-4
33 suppliers
inquiry
Yield:61063-11-4 99%
Reaction Conditions:
with acetyl chloride in methanol;ethyl acetate at 0 - 20; for 64 h;
Steps:
7.I
Example 7:; 2-Ethoxy-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazoIe-4-carboxyIic acid (candesartan) (If); Step I: 2-Amino-3-nitro-benzoic acid ethyl ester (VII); HCl IN (10 niL) [a solution of HCl IN (25 mL) was prepared by dropwise acetyl chloride (1.78 mL) in MeOH (3.22 mL) and diluting with AcOEt (20 mL)] was slowly added dropwise to ethyl 2-[(fert-butoxycarbonyl)amino]-3-n.itrobenzoate (VI) (1 g, 0.00323 mol), under N2 atmosphere, at 00C. The solution was kept at 0°C for 5', than stirred at room temperature for 64 hrs (TLC monitoring: cyclohexane/AcOEt 2:1). A saturated solution of NaHCO3 was added to the mixture and the mixture was extracted several times with AcOEt. The organic phase was dried over Na2SO4 and concentrated under reduced pressure to give the compound (VII) (0.67 g) as a yellow solid, m.p. 108°C. Yield: 99%.[1H] NMR (400 MHz, CDCl3, δ): 1.40 (t, J=7.1Hz, 3H, OCH2CH5), 4.37 (q, J=7.1ηz, 2H, OCH2CH3), 6.65 (dd, J=8.0Hz, 8.0Hz, IH) 8.25 (dd, J=7.6Hz, 1.2Hz, IH) 8.37 (dd, J=7.6Hz, 1.2Hz, IH) (aromatic protons), 8.46 (bs, 2H, NH2).
References:
WO2008/12852,2008,A1 Location in patent:Page/Page column 25; 38
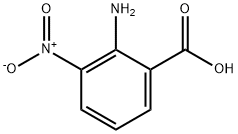
606-18-8
336 suppliers
$9.00/1g

144-55-8
1413 suppliers
$10.00/25g

75-03-6
369 suppliers
$11.00/5g

61063-11-4
33 suppliers
inquiry
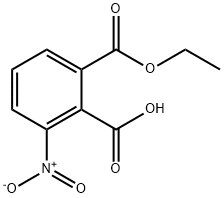
16533-45-2
99 suppliers
$7.00/250mg

61063-11-4
33 suppliers
inquiry
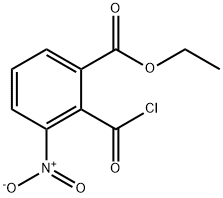
136285-66-0
2 suppliers
inquiry

61063-11-4
33 suppliers
inquiry
