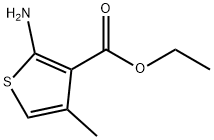
ETHYL 2-AMINO-4-METHYLTHIOPHENE-3-CARBOXYLATE synthesis
- Product Name:ETHYL 2-AMINO-4-METHYLTHIOPHENE-3-CARBOXYLATE
- CAS Number:43088-42-2
- Molecular formula:C8H11NO2S
- Molecular Weight:185.24
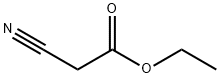
105-56-6
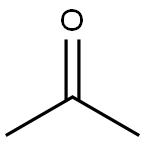
67-64-1

43088-42-2
GENERAL METHOD: Cyclohexanone (1.06 mL, 10 mmol), ethyl cyanoacetate (1.15 mL, 10 mmol), morpholine (0.90 mL, 10 mmol), and sulfur powder (0.32 g, 10 mmol) were dissolved in ethanol (10 mL), and the reaction was stirred and refluxed overnight. After completion of the reaction, the mixture was cooled to room temperature and the solvent was removed by distillation under reduced pressure. The resulting crude product was washed with cold ethanol, filtered through a sintered funnel and dried under vacuum. The dried crude product was dissolved in dichloromethane and washed with saturated saline. The organic layer was separated and concentrated under reduced pressure to afford the target compound ethyl 2-amino-4-methylthiophene-3-carboxylate (2a) in 73% yield (1.83 g).

105-56-6
461 suppliers
$10.00/5ml

67-64-1
0 suppliers
$17.30/10ml

43088-42-2
176 suppliers
$5.00/250mg
Yield:43088-42-2 76%
Reaction Conditions:
with morpholine;sulfur in ethanol at 90;Gewald reaction;
Steps:
Typical procedure for the synthesis of ethyl 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate1a (2a):
General procedure: A mixture of cyclohexanone (1.06 mL, 10 mmol), ethyl cyanoacetate (1.15 mL, 10 mmol), morpholine (0.90 mL, 10 mmol), sulphur (0.32 g, 10 mmol) in ethanol (10 mL) was stirred and refluxed for overnight. After completion of the reaction, the reaction mixture was cooled to room temperature and the solvent was removed under vacuum. The crude solid was washed with cold ethanol and filtered though sintered funnel, dried under vacuum. The crude product was dissolved in dichloromethane and washed with brine. The organic layer was collected and concentrated under low vacuum to give the compound 2a; yield: 73% (1.83 g);
References:
Nakhi, Ali;Adepu, Raju;Rambabu;Kishore, Ravada;Vanaja;Kalle, Arunasree M.;Pal, Manojit [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 13,p. 4418 - 4427] Location in patent:supporting information; experimental part

109-89-7
489 suppliers
$10.00/5g

105-56-6
461 suppliers
$10.00/5ml

67-64-1
0 suppliers
$17.30/10ml

43088-42-2
176 suppliers
$5.00/250mg