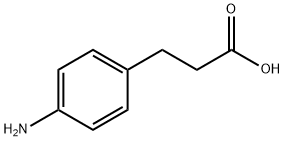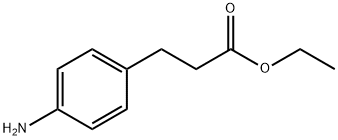
ETHYL 3-(4-AMINOPHENYL)PROPANOATE synthesis
- Product Name:ETHYL 3-(4-AMINOPHENYL)PROPANOATE
- CAS Number:7116-44-1
- Molecular formula:C11H15NO2
- Molecular Weight:193.24
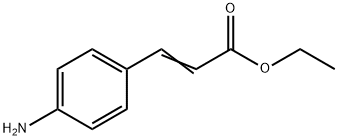
5048-82-8
166 suppliers
$135.00/10g

7116-44-1
22 suppliers
inquiry
Yield:7116-44-1 100%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol at 0 - 20; for 16 h;
Steps:
4.1.3. General method C for the synthesis of ethyl 3-(4-aminophenyl)propanoate (12)
In a solution of ethyl 4-aminocinnamate (9, 0.382 g, 2 mmol, 1 eq.) in ethanol (30 mL), 0.240 g of 10% palladium on charcoal (10% Pd/C)was added at 0 C. Then, the reaction was kept under constant stirring atroom temperature under H2 atmosphere for 16 h. The product wasfiltered through a small pad of Celite, and concentrated to aford theproduct as a yellow liquid (0.386 g, quant.). 1H NMR (300 MHz, DMSOd6)δ 10.19 (s, 2H), 7.31 (d, J = 8.6 Hz, 2H), 7.27 (d, J = 8.6 Hz, 2H),4.04 (q, J = 7.1 Hz, 2H), 2.86 (t, J = 7.4 Hz, 2H), 2.62 (t, J = 7.4 Hz, 2H),1.15 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO- d6) δ 172.0, 139.6,130.9, 129.3 (2C), 122.6 (2C), 59.8, 34.9, 29.7, 14.0.
References:
Tavares, Maurício Temotheo;de Almeida, Larissa Costa;Kronenberger, Thales;Monteiro Ferreira, Glaucio;Fujii de Divitiis, Thainá;Franco Zannini Junqueira Toledo, M?nica;Mariko Aymoto Hassimotto, Neuza;Agostinho Machado-Neto, Jo?o;Veras Costa-Lotufo, Letícia;Parise-Filho, Roberto [Bioorganic and Medicinal Chemistry,2021,vol. 35]
953-26-4
127 suppliers
$8.00/1g

7116-44-1
22 suppliers
inquiry
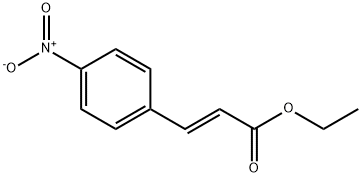
24393-61-1
26 suppliers
$48.00/5g

7116-44-1
22 suppliers
inquiry
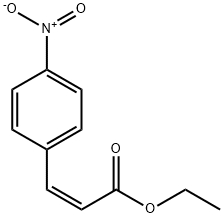
51507-21-2
1 suppliers
inquiry

7116-44-1
22 suppliers
inquiry