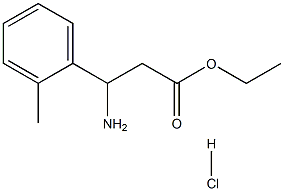
Ethyl 3-amino-3-(2-tolyl)propanoate HCl synthesis
- Product Name:Ethyl 3-amino-3-(2-tolyl)propanoate HCl
- CAS Number:21464-57-3
- Molecular formula:C12H17NO2
- Molecular Weight:207.27
Yield:21464-57-3 89%
Reaction Conditions:
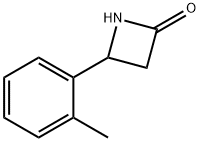
Stage #1: ethanol;4-(o-tolyl)azetidin-2-onewith hydrogenchloride for 4 h;Reflux;
Stage #2: with potassium hydroxide in water;
Steps:
4.4. Synthesis of racemic ethyl 3-amino-3-(o-tolyl)propanoate [(+/-)-3]
When (+/-)-2 (1 g) was refluxed with 22% HCl/EtOH (30 mL) for 4 h, followed by solvent evaporation (+/-)-3·HCl was obtained. Liberation of the free base with aqueous KOH (5%) afforded the desired ethyl 3-amino-3-(o-tolyl)propanoate [(+/-)-3] (1.19 g, 89%) as a colourless oil. The 1H NMR (400 MHz, DMSO) δ (ppm) data for (+/-)-3: 1.08-1.13 (3H, t, J = 7, CH3), 2.26 (3H, s, CH3), 2.43-2.51 (2H, m, CH2CH3), 3.94-4.00 (2H, m, CH2COOEt), 4.37-4.41 (1H, dd, J = 5.7; 8.2, CH), 7.05-7.41 (4H, m, Ph). Anal. calcd for C12H17NO2: C, 69.54; H, 8.27; N, 6.76. Anal. found: C, 69.66; H, 8.18; N, 6.87.
References:
Forro, Enik;Tasnadi, Gabor;Fueloep, Ferenc [Journal of Molecular Catalysis B: Enzymatic,2013,vol. 93,p. 8 - 14]
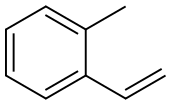
611-15-4
124 suppliers
$128.00/5g

21464-57-3
4 suppliers
inquiry

64-17-5
825 suppliers
$10.00/50g
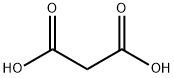
141-82-2
789 suppliers
$5.00/25g
529-20-4
341 suppliers
$5.00/5g

21464-57-3
4 suppliers
inquiry