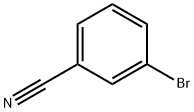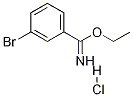
ethyl 3-bromobenzenecarboximidoate hydrochloride synthesis
- Product Name:ethyl 3-bromobenzenecarboximidoate hydrochloride
- CAS Number:57508-63-1
- Molecular formula:C9H11BrClNO
- Molecular Weight:265
Yield:57508-63-1 78%
Reaction Conditions:
with acetyl chloride at 20; for 16 h;
Steps:
49A.1 Steps 1-3: 3-(3-Bromophenyl)-l,5-diphenyl-1H-1,2,4-triazole
[00352] Acetyl chloride (16 mL, 160 mmol) was added to a solution of 3-bromo-benzonitrile (3.64 g, 20 mmol) in ethanol (14 mL, 240 mmol). The resulting mixture was stirred at room temperature for 16 h. Volatile materials were removed under vacuum to give ethyl 3- bromobenzimidate hydrochloride as a white solid (3.5 g, 78%). 1H NMR (400 MHz, CDC13): δ 12.06 (brs, 1H), 8.53 (d, J = 7.8 Hz, 1H), 8.33 (s, 1H), 7.79 (d, J = 8.2 Hz, 1H), 7.45 (t, J = 7.8 Hz, 1H), 4.91 (t, q, J = 6.9 Hz, 2H), 1.61 (t, J = 6.9 Hz, 3H). LRMS (ESI) calcd. for C9H10BrNO[M+H]+: 227.99. Found: 228.00. Benzoyl chloride (0.76 mL, 6.58 mmol) was added in dropwise to a solution of 3-bromobenzimidate hydrochloride (1.5 g, 6.58 mmol) and Et3N (0.91 mL, 6.58 mmol) in dichloromethane (50 mL). The resulting mixture was stirred at 30-40 °C for 6h. After cooling to room temperature, the reaction mixture was poured into saturated sodium bicarbonate solution. The organic layer was washed with water, brine and dried over Na2SO4 to obtain ethyl (Z)-N-benzoyl-3-bromobenzimidate as a colorless solid (2.2 g, quantitative yield) which was used for next step without further purification. LRMS (ESI) calcd. for C16H14BrNO2[M+H]+: 332.02. Found: 332.00. Ethyl (Z)-N-benzoyl-3-bromobenzimidate (2.2 g, 6.58 mmol), phenyl hydrazine hydrochloride (1.9 g, 13.16 mmol), Et3N (1.8 mL, 13.16 mmol) were taken in dichloromethane (50 mL) and heated at 30-40 °C for 4h. Cooled to room temperature and diluted with water and followed by a usual work up with dichloromethane. Evaporation of the solvent followed by silica gel column chromatography using hexanes:ethyl acetate afforded 3-(3-bromophenyl)-l ,5-diphenyl-1H-l ,2,4-triazole as a white solid (1.8 g, 73%). 1H NMR (400 MHz, CDC13): δ 8.40 (t, J = 1.8 Hz, 1H), 8.14 (dd, J = 1.3 Hz, 8.2 Hz, 1H), 7.54- 7.52 (m, 3H), 7.43-7.41 (m, 9H). LRMS (ESI) calcd. for C20H14BrN3 [M+H]+: 376.25. Found: 376.00.
References:
WO2015/191630,2015,A1 Location in patent:Paragraph 00352