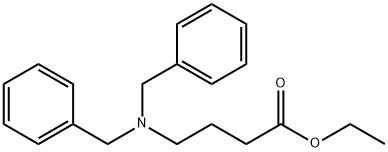
Ethyl 4-(Dibenzylamino)butanoate synthesis
- Product Name:Ethyl 4-(Dibenzylamino)butanoate
- CAS Number:94911-63-4
- Molecular formula:C20H25NO2
- Molecular Weight:311.42
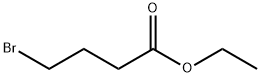
2969-81-5
458 suppliers
$5.00/1g
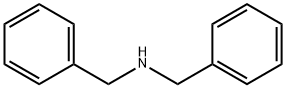
103-49-1
473 suppliers
$5.00/25g

94911-63-4
30 suppliers
$45.00/50mg
Yield:94911-63-4 98%
Reaction Conditions:
with potassium carbonate;potassium iodide in N,N-dimethyl-formamide at 80;
Steps:
1.33
33) Preparation of aldehyde and acid; Preparation of 4-Dibenzylamino-butyric acid ethyl ester (Intermediate):; [Show Image] To a solution of dibenzylamine (962 μL, 5 mmol., 1 equiv.) in 2.5 ml of anhydrous DMF was added 715 μL (5 mmol., I equiv.) of ethyl 4-bromobutyrate, 1.38 g (10 mmol., 2 equiv.) of potassium carbonate and 83 mg (0.5 mmol., 0.1 equiv.) of potassium iodide. The resulting mixture was heated at 80°C overnight. The slurry was partitioned with water and ethyl acetate. The aqueous layer was extracted twice with ethyl acetate. The organic layer was dried over sodium sulphate, evaporated and used without further purification. Yellow oil (98%) 1H NMR (400 MHz, CDCl3) δ 7.26 (m, 10H, Ph), 4.10 (q, J = 7.1 Hz, 2 H, OCH2CH3), 3.56 (s, 4H, NCH2Ph), 2.45 (t, J = 6.8 Hz, 2H, NCH2CH2CH2COOEt), 2.32 (t, J = 7.5 Hz, 2H, NCH2CH2CH2COOEt), 1.83 (m, 2H, NCH2CH2CH2COOEt), 1.22 (t, J = 7.1 Hz, 3H, OCH2CH3), LC/MS (ES+) m/z 313.3 (M+H)+
References:
EP1997805,2008,A1 Location in patent:Page/Page column 90
7425-53-8
56 suppliers
$16.00/250mg

103-49-1
473 suppliers
$5.00/25g

94911-63-4
30 suppliers
$45.00/50mg
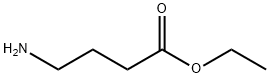
5959-36-4
27 suppliers
inquiry

100-44-7
659 suppliers
$13.50/250G

94911-63-4
30 suppliers
$45.00/50mg