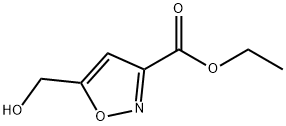
ETHYL 5-(HYDROXYMETHYL)ISOXAZOLE-3-CARBOXYLATE synthesis
- Product Name:ETHYL 5-(HYDROXYMETHYL)ISOXAZOLE-3-CARBOXYLATE
- CAS Number:123770-62-7
- Molecular formula:C7H9NO4
- Molecular Weight:171.15
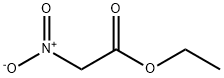
626-35-7

107-19-7

123770-62-7
The general procedure for the synthesis of ethyl 5-hydroxymethylisoxazole-3-carboxylate from ethyl nitroacetate and 2-propyn-1-ol was as follows: intermediate 4- Preparation of ethyl 5-(hydroxymethyl)isoxazole-3-carboxylate. In an Ace pressure tube, 2-propyn-1-ol (1.0 mL, 16.76 mmol) and ethyl nitroacetate (3.79 mL, 33.52 mmol) were dissolved in ethanol (23.5 mL), followed by the addition of 1,4-diazabicyclo[2.2.2]octane (DABCO, 0.194 g, 1.68 mmol). The reaction tube was sealed and the reaction was heated at 80 °C for 72 hours. After completion of the reaction, it was cooled to room temperature and the reaction mixture was concentrated to dryness under reduced pressure. The resulting residue was purified by fast column chromatography on silica gel, the eluent being a mixture of dichloromethane and methanol (methanol v/v fraction 0% to 6%), resulting in ethyl 5-(hydroxymethyl)isoxazole-3-carboxylate 2.32 g in 81% yield as an oil.ESI/APCI (+): m/z 172 ([M+H]+).
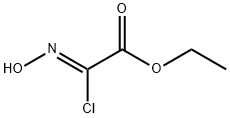
95080-93-6
1 suppliers
inquiry

107-19-7
0 suppliers
$12.00/25g

123770-62-7
72 suppliers
$39.00/100mg
Yield: 81%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 25;
Steps:
ethyl 5-(hydroxymethyl)isoxazole-3-carboxylate
In a 100 mL round-bottomed flask (Z)-ethyl 2-chloro-2-(hydroxyimino)acetate (164.96 mmol) and propargyl alcohol (824.82 mmol) were taken in tetrahydrofuran (200 mL) to give a yellow solution. Reaction mass was cooled to 0 °C and triethyl amine (197.96 mmol) in tetrahydrofuran (100 mL) was added slowly over 45 min. Reaction mass was then allowed to cool to 25 °C and stirred overnight. The reaction mass was diluted with brine (250 mL) and extracted with dichloromethane (3x 300 mL). Dichloromethane layers were collected and dried over sodium sulphate and concentrated to give crude mass which on purification over combiflash gave ethyl 5-(hydroxymethyl)isoxazole-3-carboxylate (81 %) as pure liquid. 1H MR (300 MHz, DMSO-i) δ ppm 3.82 (t, J=7.06 Hz, 3 H) 6.74 - 6.98 (m, 2 H) 7.08 - 7.18 (m, 2 H) 8.25 - 8.39 (m, 1 H) 9.25 (s, 1 H) MS (ES+), (M+H)+ = 172.26 for C9H9N04
References:
FOUNDATION FOR NEGLECTED DISEASE RESEARCH;RATAN KALE, Ramesh;MADHAVAPEDDI, Prashanti;RAGHUNATH GHORPADE, Sandeep;VITHALRAO BELLALE, Eknath;GANPAT KALE, Manoj;RAICHURKAR, Anandkumar;LANDGE, Sudhir;DOUGLAS COWEN, Scott;ANTHONY READ, Jonathan;IYER, Pravin;NARAYANAN, Shridhar WO2015/181799, 2015, A1 Location in patent:Page/Page column 16; 17

626-35-7
267 suppliers
$5.00/1g

107-19-7
0 suppliers
$12.00/25g

123770-62-7
72 suppliers
$39.00/100mg

107-19-7
0 suppliers
$12.00/25g
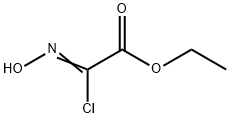
14337-43-0
223 suppliers
$9.00/1g

123770-62-7
72 suppliers
$39.00/100mg

107-19-7
0 suppliers
$12.00/25g
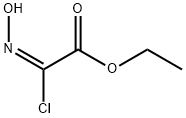
861135-87-7
4 suppliers
$681.00/500g

123770-62-7
72 suppliers
$39.00/100mg
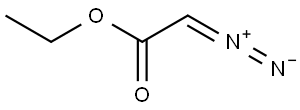
623-73-4
151 suppliers
$26.69/5g

107-19-7
0 suppliers
$12.00/25g

123770-62-7
72 suppliers
$39.00/100mg