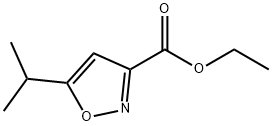
Ethyl 5-isopropyl-3-isoxazolecarboxylate synthesis
- Product Name:Ethyl 5-isopropyl-3-isoxazolecarboxylate
- CAS Number:91240-30-1
- Molecular formula:C9H13NO3
- Molecular Weight:183.2
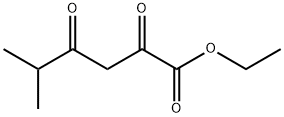
64195-85-3
47 suppliers
$32.39/1g

91240-30-1
32 suppliers
inquiry
Yield:91240-30-1 98%
Reaction Conditions:
with hydroxylamine hydrochloride in ethanol at 15 - 25; for 1 h;Heating / reflux;
Steps:
F.612 Example 612; N-(5-fluoro-2,4-dimethoxyphenyl)-N'-(5-isopropylisoxazol-3-yl)urea.
Sodium (1.33 g, 57.8 mmol) is added to EtOH (30 mL) and at 0° C. and 3-methyl-2-butanone (6.2 mL, 57.7 mmol) and diethyl oxalate (7.9 mL, 57.8 mmol) are added. The resulting solid is allowed to stand for 1 h and then heated to 80° C. for 0.75 h. The reaction is cooled to RT and acidified to pH 2 with dilute sulfuric acid. Water (30 mL) is then added and the product extracted with EtOAc (2×50 mL), dried (MgSO4), and the solvent is removed to give ethyl 5-methyl-2,4-dioxohexanoate as an orange oil (9.74 g, 90% yield). MS (ESI) for C9H14O4 m/z 187 (M+H)+. Hydroxylamine hydrochloride (10.9 g, 156.3 mmol) is added to ethyl 5-methyl-2,4-dioxohexanoate (9.7 g, 52.1 mmol) stirring in EtOH (200 mL) at RT. The reaction is heated to reflux for 1 h then cooled to RT and the solvent is removed. The residue is diluted with H2O (50 mL) and extracted with EtOAc (3×50 mL) and dried (MgSO4) and the solvent is removed to give ethyl 5-isopropylisoxazole-3-carboxylate as an orange oil (9.39 g, 98% yield). MS (ESI) for C9H13NO3 m/z 184 (M+H)+. Hydrazine hydrate (1.2 g, 24.7 mmol) is added to ethyl 5-isopropylisoxazole-3-carboxylate (3.0 g, 16.4 mmol) stirring in EtOH (30 mL) and the reaction is heated to 60° C. for 3 h. The reaction is cooled to RT and the solid filtered (157 mg). The filtrate is concentrated and the solid collected by filtering from petroleum ether to give 5-isopropylisoxazole-3-carbohydrazide as an off-white solid (2.46 g, 88% yield). MS (ESI) for C7H11N3O3 m/z 170 (M+H)+. An aqueous solution of NaNO2 (1.1 g in 10 mL H2O) is added dropwise to 5-isopropylisoxazole-3-carbohydrazide (2.1 g, 12.5 mmol) in conc. HCl (60 mL). The reaction is stirred for 1.5 h and diluted with H2O (25 mL). The precipitate is filtered, washed with H2O (50 mL) and dried to give 5-isopropylisoxazole-3-carbonyl azide as a white solid (1.17 g, 52% yield). The aqueous is extracted with EtOAc (3×50 mL) to give a second crop of product (0.85 g, total yield=90% yield). Example 612 is obtained according to Method F, making non-critical modifications. Yield 71%. HRMS (ESI) calcd for C15H18FN3O4+H 324.1359 found 324.1354.
References:
US2003/236287,2003,A1 Location in patent:Page 45, 46
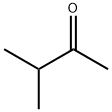
563-80-4
315 suppliers
$18.00/25mL

91240-30-1
32 suppliers
inquiry