Cyclohexanecarboxylic acid ethyl ester synthesis
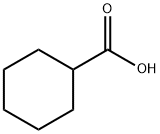
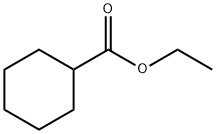
- Product Name:Cyclohexanecarboxylic acid ethyl ester
- CAS Number:3289-28-9
- Molecular formula:C9H16O2
- Molecular Weight:156.22
Yield:3289-28-9 98%
Reaction Conditions:
with sulfuric acid at 76; for 7 h;Reagent/catalyst;Concentration;Time;
Steps:
3.2.3.1. Ethyl cyclohexanecarboxylate
Cyclohexanecarboxylic acid (1.28 g, 0.1 mmol), anhydrous ethyl alcohol (4.6 g, 1.0 mmol), and thecatalyst (300 mg) were mixed in a 30 mL flask at °C and stirred at the same temperature for 23 h. Afterthe reaction mixture was cooled down to room temperature it was filtered through Celite. The ethylalcohol was evaporated under reduced pressure to give an oil that was diluted with diethyl ether(50 mL) and the solution was washed twice with deionized water. The ether layer was separated anddried over anhydrous magnesium sulfate. After removal of insoluble materials by filtration, the ethersolution was evaporated to give the ethyl cyclohexanecarboxylate. When the sulfonated carbons(300 mg), Amberlyst-15 (300 mg), and conc, sulfuric acid (100 mg), conc, sulfuric acid (18.2 mg),were used as a catalyst in the reaction, the yields of the ester in each case were 92% (SC-II), 85%(SC-I), 84% (recovered SC-I), 83% (Amberlyst-15), 98% (H2SO4, 100 mg), and 88% (H2SO4, 18.2 mg),respectively.
References:
Lee, Duckhee [Molecules,2013,vol. 18,# 7,p. 8168 - 8180]
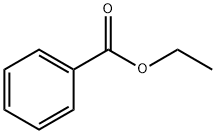
93-89-0
527 suppliers
$13.00/100g

3289-28-9
115 suppliers
$13.00/1g
2150-37-0
297 suppliers
$6.00/5g
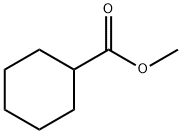
4630-82-4
212 suppliers
$6.00/25g

3289-28-9
115 suppliers
$13.00/1g