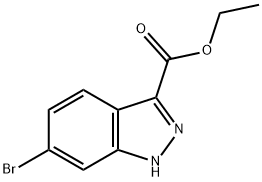
ETHYL 6-BROMO-1H-INDAZOLE-3-CARBOXYLATE synthesis
- Product Name:ETHYL 6-BROMO-1H-INDAZOLE-3-CARBOXYLATE
- CAS Number:885272-94-6
- Molecular formula:C10H9BrN2O2
- Molecular Weight:269.09
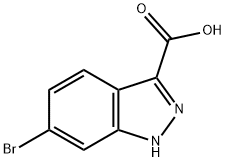
660823-36-9

885272-94-6
To a solution of 6-bromoindazole-3-carboxylic acid (4.4 g, 18.25 mmol) in ethanol (100 mL) was slowly added thionyl chloride (666 mL, 91 mmol), followed by heating and refluxing the reaction mixture for 3 hours. Upon completion of the reaction, the reaction mixture was concentrated under reduced pressure and purified by fast column chromatography to afford ethyl 6-bromo-1H-indazole-3-carboxylate (2.1 g, 39% yield) as a tan solid. Mass spectrum (ESI) m/z: 270.9 (M + H). NMR hydrogen spectrum (400MHz, CDCl3) δ 8.08 (d, J = 8.6Hz, 1H), 7.95 (s, 1H), 7.44 (d, J = 8.6Hz, 1H), 4.57 (q, J = 7.0Hz, 2H), 1.51 (t, J = 7.2Hz, 3H).

660823-36-9
139 suppliers
$31.00/100mg

885272-94-6
72 suppliers
$45.00/100mg
Yield:885272-94-6 39%
Reaction Conditions:
with thionyl chloride in ethanol; for 3 h;Reflux;
Steps:
intermediate 112B. Preparation of ethyl 6-bromo4H-indazole-.3carboxylate.
To a solution of Intermediate ii 2A (4.4 g, 18.25 mmol) in EtOH (100 mL) was added SOCI2 (666 mL, 91 rnmol) and refiuxed for 3 h, The reaction mixture was concentrated and purified using flash colunm chromatography to afford ethyl 6-bromoiH4ndazole3carboxylate (Intermediate 1 12B) (2.1 g, 39%) as tan solid. MS(ESI) m/z:270.9 (M±H). ‘FT NMR (400 MFTz, CDCI3) 3 808 (d, J=8.6 Hz. 1H). 7.95 (s. 1H). 7.44 (d, J8.6 Hz. 1H), 457 (q, .J7.0 Hz. 2H), 1.51 (t, .1=7.2 Hz. 3H).
References:
WO2017/123860,2017,A1 Location in patent:Page/Page column 248

660823-36-9
139 suppliers
$31.00/100mg

64-17-5
825 suppliers
$10.00/50g

885272-94-6
72 suppliers
$45.00/100mg
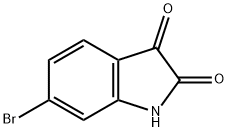
6326-79-0
277 suppliers
$6.00/1g

885272-94-6
72 suppliers
$45.00/100mg