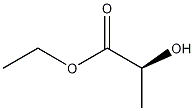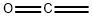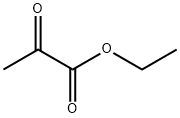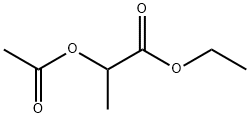
ethyl acetyl lactate synthesis
- Product Name:ethyl acetyl lactate
- CAS Number:2985-28-6
- Molecular formula:C7H12O4
- Molecular Weight:160.17
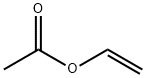
108-05-4
589 suppliers
$4.00/1000g

64-17-5
757 suppliers
$10.00/50g
201230-82-2
1 suppliers
inquiry

2985-28-6
14 suppliers
inquiry
Yield:2985-28-6 76%
Reaction Conditions:
Stage #1: vinyl acetate;ethanolwith pyridine in toluene;Inert atmosphere;
Stage #2: carbon monoxidewith dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane;pyridine;toluene-4-sulfonic acid;palladium dichloride in toluene at 100; under 37503.8 Torr; for 24 h;
Steps:
7-8
In the reaction vessel containing these catalysts, under a nitrogen stream,Vinyl acetate as vinyl carboxylate (manufactured by Wako Pure Chemical Industries, Ltd .; 0.75 mL, 8.1 mmol) and Table 1Primary or secondary alcohol (0.81 mL, 20 mmol) shown in the above, toluene as a solvent (manufactured by Wako Pure Chemical Industries, Ltd .; 6.75 mL), and a nitrogen atom-containing heterocyclic compound as a catalyst.Pyridine (manufactured by Wako Pure Chemical Industries, Ltd .; 0.0275 mL,340.2 μmol) was added at the ratio shown in Table 1 to obtain a reaction solution.The amount of palladium chloride used in Examples 1 to 8 is 0.002 mol parts with respect to 1 mol part of vinyl acetate.The amount of the organic phosphine ligand used is 2 mol parts with respect to 1 mol part of palladium chloride.The amount of PTSA (p-toluenesulfonic acid monohydrate) used is 0.395 mol parts of acid point of PTSA with respect to 1 mol part of palladium chloride.The amount of pyridine used is 20 mol parts with respect to 1 mol part of palladium chloride.The amount of toluene used is 0.83 liters (the amount at which the concentration of vinyl acetate in the reaction solution is 1.2 mol / liter) with respect to 1 mol part of the primary or secondary alcohol.Then, using carbon monoxide gas, pressurization-depressurization in the reaction vessel was repeated 3 times between atmospheric pressure and 1 MPa, and the gas in the reaction vessel was replaced with carbon monoxide gas.Then, using carbon monoxide gas, the pressure inside the reaction vessel was adjusted to the pressure shown in Table 2, and the reaction solution was stirred while stirring.The reaction was carried out at the reaction temperature shown in Table 2 for the reaction time shown in Table 2 to produce an ester compound.
References:
JP2021/183589,2021,A Location in patent:Paragraph 0066-0076

92828-47-2
9 suppliers
$244.00/500mg

2985-28-6
14 suppliers
inquiry