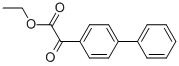
ethyl alpha-oxo[1,1'-biphenyl]-4-acetate synthesis
- Product Name:ethyl alpha-oxo[1,1'-biphenyl]-4-acetate
- CAS Number:6244-53-7
- Molecular formula:C16H14O3
- Molecular Weight:254.28
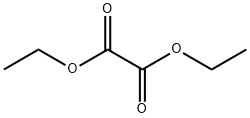
95-92-1
437 suppliers
$5.00/5G
![ethyl alpha-oxo[1,1'-biphenyl]-4-acetate](/CAS/GIF/6244-53-7.gif)
6244-53-7
17 suppliers
$252.00/1g
Yield:6244-53-7 55%
Reaction Conditions:
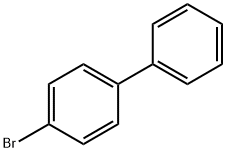
Stage #1: 4-bromo-1,1'-biphenylwith magnesium in tetrahydrofuran at 20; for 0.5 - 0.666667 h;Heating / reflux;
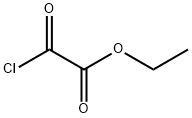
Stage #2: oxalic acid diethyl ester in tetrahydrofuran at -78 - 10; for 1 h;
Steps:
8
To a three-neck round bottom flask containing Mg turnings (1.2 equiv.) equipped with a magnetic stirrer and dimroth condenser was added a solution of alkyl bromide 90 (1 equiv.) in anhydrous THF via syringe and external heating under argon atmosphere. The reaction mixture was refluxed gently for 30-40 min. and then it was cooled to room temperature, before conveying it to a dropping funnel. The Grignard reagent was added dropwise to a solution of diethyl oxalate (1.5 equiv.) in THF at -78°C. The reaction mixture was warmed to 1O0C within 1 hour and then was quenched by the addition of saturated ammonium chloride solution. The organic layer was separated, the aqueous layer was extracted with diethyl ether and the combined organic layer was washed with brine, dried over MgSO4 and evaporated. The residue was purified by flash column chromatography on silica gel (diethyl ether-hexane) to give pure compound 91 in 55-65% yields.Selected data of synthesized α-Keto-esters (91). Ethyl 2-(biphenyl-4-yl)-2-oxoacetate (91.1).1H NMR (500 MHz, CDCl3) δ 8.09 (d, J = 8.5 Hz, 2H)5 7.74 (d, J = 8.5 Hz5 2H)5 7.65 (d, J =7.2 Hz5 2H)5 7.49 (t, J = 7.2 Hz, 2H)5 7.43 (t5 J = 7.2 Hz, IH), 4.47 (q, J = 7.5 Hz, 2H)5 1.45(t, J = 7.5 Hz5 3H).
References:
WO2008/13963,2008,A2 Location in patent:Page/Page column 96

64-17-5
815 suppliers
$10.00/50g
29079-00-3
136 suppliers
$9.00/250mg
![ethyl alpha-oxo[1,1'-biphenyl]-4-acetate](/CAS/GIF/6244-53-7.gif)
6244-53-7
17 suppliers
$252.00/1g
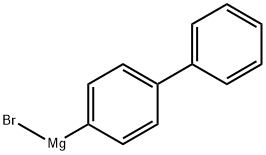
3315-91-1
70 suppliers
$153.00/50mL

95-92-1
437 suppliers
$5.00/5G
![ethyl alpha-oxo[1,1'-biphenyl]-4-acetate](/CAS/GIF/6244-53-7.gif)
6244-53-7
17 suppliers
$252.00/1g