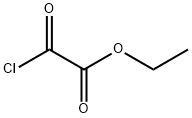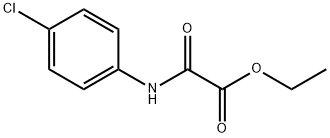
ethyl N-(4-chlorophenyl)-2-oxoglycinate synthesis
- Product Name:ethyl N-(4-chlorophenyl)-2-oxoglycinate
- CAS Number:5397-14-8
- Molecular formula:C10H10ClNO3
- Molecular Weight:227.64
Yield:5397-14-8 100%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 20; for 6 h;
Steps:
4.1.1. N1-(4-Chlorophenyl)-N2-(piperidin-4-yl)oxalamide (3)
To a stirred solution of p-choroaniline (1) (14.0 g, 110 mmol) in THF (146 mL) were added ethyl chloroglyoxylate (8.13 mL, 73.2 mmol) and triethylamine (Et3N) (15.2 mL, 110 mmol) at 0 °C. The mixture was stirred for 6 h at room temperature. After the precipitate was filtrated off, the filtrate solution was concentrated under reduced pressure. The residue was dissolve in EtOAc, and washed with 1 M HCl, saturated NaHCO3 and brine, then dried over MgSO4. Concentration under reduced pressure gave the crude ethyl oxalamate, which was used without further purification. To a solution of the above ethyl oxalamate (1.27 g, 5.25 mmol) in EtOH (13.0 mL) were added Et3N (1.46 mL, 10.5 mmol) and 4-amino-1-benzylpiperidine (2.97 mL, 15.8 mmol). The reaction mixture was stirred for 3 h at 150 °C under microwave irradiation. After being cooled to room temperature, the crystal was collected and washed with cold EtOH and n-hexane, and dried under reduced pressure to provide the corresponding amide (1.58 g, 81% yield) as colorless crystals. To a stirred solution of S1 (1.46 g, 3.90 mmol) in CH2Cl2 (39.0 mL) was added dropwise 1-chloroethyl chloroformate (0.860 mL, 7.80 mmol) at 0 °C. After being stirred at room temperature for 30 min, the mixture was refluxed for 1 h. After concentration under reduced pressure, the residue was dissolved in MeOH and then refluxed for 1 h. After concentration under reduced pressure, the residue was diluted with EtOAc and washed with saturated NaHCO3 and brine, then dried over MgSO4. After concentration under reduced pressure, the residue was washed with cold EtOAc, and dried under reduced pressure to provide the title compound 3 (778 mg, 71% yield) as white powder.Comment1H NMR (400 MHz, CDCl3) δ 1.39-1.52 (m, 2H), 1.92-2.01 (m, 2H), 2.67-2.79 (m, 2H), 3.06-3.19 (m, 2H), 3.83-3.95 (m, 1H), 7.34 (d, J = 8.80 Hz, 2H), 7.44 (d, J = 7.64 Hz, 1H), 7.59 (d, J = 8.80 Hz, 2H), 9.28 (s, 1H); 13C NMR (125 MHz, CDCl3) δ 33.0 (2C), 45.2 (2C), 47.9, 21.0 (2C), 129.3 (2C), 130.5, 135.0, 157.6, 158.8; HRMS (ESI), m/z calcd for C13H17ClN3O2 (MH+) 282.1004, found 282.1002.
References:
Narumi, Tetsuo;Arai, Hiroshi;Yoshimura, Kazuhisa;Harada, Shigeyoshi;Nomura, Wataru;Matsushita, Shuzo;Tamamura, Hirokazu [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 22,p. 6735 - 6742] Location in patent:experimental part
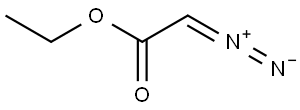
623-73-4
153 suppliers
$27.30/25ML
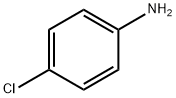
106-47-8
495 suppliers
$5.00/5G

5397-14-8
19 suppliers
$59.00/5mg