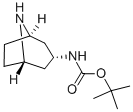
exo-3-Boc-aminotropane synthesis
- Product Name:exo-3-Boc-aminotropane
- CAS Number:132234-68-5
- Molecular formula:C12H22N2O2
- Molecular Weight:226.32
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

132234-68-5
75 suppliers
$45.00/50mg
Yield: 97%
Reaction Conditions:
with hydrogen;10% palladium on activated carbon in methanol at 40 - 50; under 760.051 Torr; for 1.5 h;
Steps:
8.b EXAMPLE 8; 3-Methoxythieno[2,3-b]pyridine-2-carboxylic acid [8-(4-chlorobenzyl)-8-azabicyclo[3.2.1]oct-3β-yl]amide hydrochloride; b) ter-Butyl-N-(8-azabicyclo[3.2.1]oct-3 β-yl)carbamate
1.6 g of ter-butyl-N-[8-(phenylmethyl)-8-azabicyclo[3.2.1]oct-3β-yl]carbamate (5.05 mmol, 1 eq.) dissolved in 80 ml of methanol is hydrogenated at atmospheric pressure at 45-50° C. in the presence of 0.1 g of 10% palladium-coated charcoal for 90 min. The catalyst is filtered, rinsed with methanol and vacuum evaporated. 1.1 g (97%) of the secondary amine product is obtained. FP=123° C. NMR (1H, CDCl3): 1.30 (m; 2H), 1.41 (s; 9H) , 1.74 (m; 4H), 1.88 (m; 2H), 3.52 (m; 2H, H1 and H5), 3.78 (m; H1, H3), 4.34 (m; 1H; NH-carbamate)
References:
Imbert, Thierry;Monse, Barbara;Koek, Wouter US2005/80085, 2005, A1 Location in patent:Page/Page column 9
![3-AMINO-8-BENZYL-8-AZABICYCLO[3.2.1]OCTANE (3-EXO)-](/CAS/GIF/76272-36-1.gif)
76272-36-1
83 suppliers
$42.40/1mg:

132234-68-5
75 suppliers
$45.00/50mg

24424-99-5
871 suppliers
$13.50/25G

132234-68-5
75 suppliers
$45.00/50mg
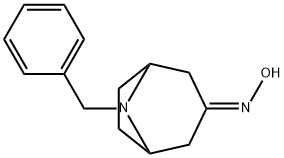
76272-34-9
49 suppliers
$140.00/50 mg

132234-68-5
75 suppliers
$45.00/50mg
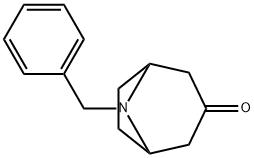
28957-72-4
210 suppliers
$27.00/1g

132234-68-5
75 suppliers
$45.00/50mg