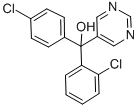
Fenarimol synthesis
- Product Name:Fenarimol
- CAS Number:60168-88-9
- Molecular formula:C17H12Cl2N2O
- Molecular Weight:331.2
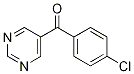
103686-55-1
10 suppliers
inquiry
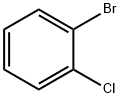
694-80-4
404 suppliers
$5.00/10g

60168-88-9
104 suppliers
$140.00/250mg
Yield:60168-88-9 20%
Reaction Conditions:
Stage #1:2-bromo-1-chlorobenzene with magnesium;ethylene dibromide in tetrahydrofuran for 1.58333 h;
Stage #2:(4-chlorophenyl)(pyrimidin-5-yl)methanone with zinc(II) chloride in tetrahydrofuran at 20; for 0.5 h;
Steps:
3.3
To a suspension of magnesium turnings (1.00 g, excess) in THF (20 mL) was added 1,2-dibromoethane (0.05 mL). After 5 min, o-bromochlorobenzene (960 mg, 5 mmol) was added in one portion, causing slight warming as the oxidative addition initiated. After 90 min. a menthol-phenanthroline titration of this solution indicated 100% insertion. This grey solution was treated with ZnCl2 (680 mg, 5 mmol) and then with 23 (1.1 g, 5 mmol). The resulting yellow-brown solution was stirred at rt for 30 min during which all of 23 was consumed according to TLC. The mixture was washed with NH4C1, sat. aq. NaHC03, and the organic layer was evaporated under reduced pressure to a brown gum. This residue was subjected to chromatography over 15 g of silica gel, eluting with 3: 1 hexane-EtOAc going to 1: 1 hexane-EtOAc. Evaporation of the eluant between 100 mL and 250 mL afforded 19 as a white solid (330 mg, 20%). Mp = 115-116 °C; 1H NMR (300 MHz, CDC13) δ ppm 9.00 (s, 1H), 8.48 (s, 2H), 8.20-7.08 (m, 8H, Ar); 13C NMR (75 MHz, CDC13) δ 160.1, 156.7, 138.7, 138.1, 137.5, 132.8, 132.3, 128.9, 128.4,128.2, 127.4, 118.4, 118.5, 80.1.
References:
REGENTS OF THE UNIVERSITY OF MINNESOTA;VINCE, Robert;VARTAK, Ashish P. WO2012/128965, 2012, A2 Location in patent:Page/Page column 16-17
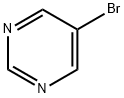
4595-59-9
463 suppliers
$6.00/1g
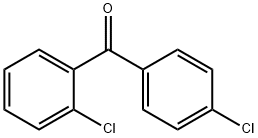
85-29-0
179 suppliers
$10.00/5g

60168-88-9
104 suppliers
$140.00/250mg