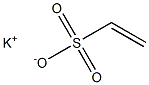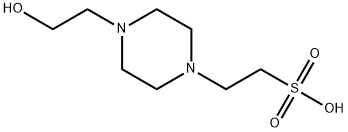
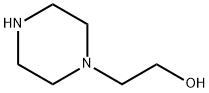
HEPES synthesis
- Product Name:HEPES
- CAS Number:7365-45-9
- Molecular formula:C8H18N2O4S
- Molecular Weight:238.3
103-76-4
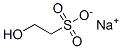
1562-00-1

7365-45-9
The general procedure for the synthesis of 4-hydroxyethylpiperazineethanesulfonic acid from N-hydroxyethylpiperazine and sodium 2-hydroxyethylsulfonate is as follows: 0.5 mole of N-hydroxyethylpiperazine, 0.565 mole of sodium 2-hydroxyethylsulfonate, and 500 ml of methanol were added to a 1000 ml four-necked flask fitted with a refluxing condenser tube, and the condensation reaction was carried out under sufficient stirring. The reaction was first carried out at 50 °C for 2.5 minutes, then gradually heated to reflux and continued for 3.0 hours. The reaction was cooled after completion of the reaction to give a reaction mixture containing 4-hydroxyethylpiperazine ethanesulfonate. The yield of 4-hydroxyethylpiperazine ethanesulfonate was 93.6% as analyzed by high performance liquid chromatography. A 20 liter solution of 4-hydroxyethylpiperazine ethanesulfonate at a mass concentration of 15 wt% was diluted to 5 wt% with deionized water under stirring, followed by removal of sodium chloride by dialysis. Then the purified HEPES product was obtained by evaporation concentration, cooling crystallization and filtration. Finally, the product was washed once with anhydrous methanol and dried under vacuum to obtain purified HEPES.A sample of purified HEPES was dissolved and tested for purity, which showed a purity of 99.9%, a yield of 85.6%, a pH of 6.46, and a concentration of chloride ions of 6.8 μg/g.

103-76-4
532 suppliers
$6.00/25g
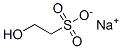
1562-00-1
414 suppliers
$5.00/25g

7365-45-9
717 suppliers
$6.00/25g
Yield:7365-45-9 85.6%
Reaction Conditions:
Stage #1: 1-(2-hydroxyethyl)piperazine;sodium 2-hydroxyethanesulfonate in methanol at 50; for 5.5 h;
Stage #2: with 5percent by weight of raffinate in water;Solvent;Temperature;
Steps:
2; 5
0.5 mol of N-hydroxyethylpiperazine, 0.565 mol of sodium 2-hydroxyethanesulfonate and 500 mL of methanol were charged into a 1000 mL four-necked flask equipped with a reflux tube and subjected to a condensation reaction with sufficient stirring;First reacted at 50 for 2.5 hours, then gradually heated to reflux and continued to react for 3.0 hours;And then cooled to obtain a reaction mother liquor containing 4-hydroxyethylpiperazineethanesulfonate.The yield of sodium 4-hydroxyethylpiperazineethanesulfonate was calculated to be 93.6% by high performance liquid chromatography. 20 L of 4-hydroxyethylpiperazineethanesulfonate,Mass concentration of 15wt% raffinate, in the stirring effect,Diluted with deionized water to a concentration of 5 wt% by dialysis dialysis to remove sodium chloride,And finally concentrated by evaporation, cooling crystallization, filtration to get pure HEPES products,And finally washed once with anhydrous methanol, and dried by vacuum to obtain purified HEPES. The purified HEPES sample was formulated as a solution and then tested for purity:The purified HEPES sample was prepared as a solution and then tested for purity, 99.9%, yield 85.6%, pH = 6.46, Cl- concentration 6.8 μg / g.
References:
CN106905262,2017,A Location in patent:Paragraph 0034; 0035; 0047

103-76-4
532 suppliers
$6.00/25g
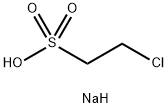
15484-44-3
343 suppliers
$10.00/25g

7365-45-9
717 suppliers
$6.00/25g

103-76-4
532 suppliers
$6.00/25g
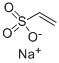
3039-83-6
324 suppliers
$14.00/5g

7365-45-9
717 suppliers
$6.00/25g

103-76-4
532 suppliers
$6.00/25g
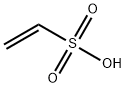
1184-84-5
62 suppliers
$45.00/10mg

7365-45-9
717 suppliers
$6.00/25g