
Hydrogen fluoride synthesis
- Product Name:Hydrogen fluoride
- CAS Number:7664-39-3
- Molecular formula:FH
- Molecular Weight:20.01
Anhydrous hydrogen fluoride is manufactured by treating fluorspar (fluorite, CaF2) with concentrated sulfuric acid in heated kilns. The gaseous HF evolved is purified by distillation, condensed as liquid anhydrous HF, and stored in steel tanks and cylinders.
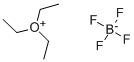
368-39-8
260 suppliers
$39.39/25ml
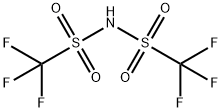
82113-65-3
267 suppliers
$25.00/1g
![Oxonium, triethyl-, salt with 1,1,1-trifluoro-N-[(trifluoromethyl)sulfonyl]methanesulfonamide (1:1)](/CAS/20211123/GIF/945614-34-6.gif)
945614-34-6
0 suppliers
inquiry

7664-39-3
443 suppliers
$21.00/25ml
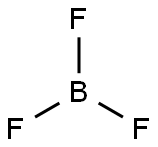
7637-07-2
0 suppliers
$35.29/10ML
Yield:945614-34-6 100%
Reaction Conditions:
at 75 - 80; for 3 h;
Steps:
3
Example 3Triethyloxonium bis(trifluoromethylsulfonyl)imide A mixture of 3.7 g (19.47 mmol) of triethyloxonium tetrafluoroborate and 5.47 g (19.46 mmol) of bis(trifluoromethylsulfonyl)imide is heated to 75-80° C. (temperature of the oil bath) and stirred for three hours under a nitrogen atmosphere. Volatile constituents are pumped off over the course of one hour under reduced pressure (7 Pa) at 75-80° C. (temperature of the oil bath), giving 7.46 g of an oil. The yield of triethyloxonium bis(trifluoromethylsulfonyl)imide is virtually quantitative. The product was investigated by NMR spectroscopy.1H NMR spectrum, ppm: 1.51 t (3CH3), 4.68 q (3CH2); J3H,H=7.1 Hz.19F NMR spectrum, ppm: -78.98 s
References:
US2009/36628,2009,A1 Location in patent:Page/Page column 10
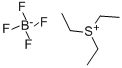
368-40-1
16 suppliers
inquiry

82113-65-3
267 suppliers
$25.00/1g
![Oxonium, triethyl-, salt with 1,1,1-trifluoro-N-[(trifluoromethyl)sulfonyl]methanesulfonamide (1:1)](/CAS/20211123/GIF/945614-34-6.gif)
945614-34-6
0 suppliers
inquiry

7664-39-3
443 suppliers
$21.00/25ml

7637-07-2
0 suppliers
$35.29/10ML
70387-18-7
0 suppliers
inquiry
513-81-5
124 suppliers
$45.00/1g
1400258-79-8
1 suppliers
inquiry

7664-39-3
443 suppliers
$21.00/25ml
75-08-1
248 suppliers
$19.89/25ml
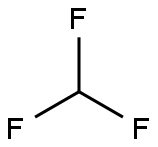
75-46-7
0 suppliers
$150.00/50g
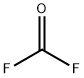
353-50-4
0 suppliers
inquiry

124-38-9
133 suppliers
$214.00/14L

7664-39-3
443 suppliers
$21.00/25ml

7732-18-5
506 suppliers
$13.50/100ML
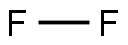
7782-41-4
0 suppliers
inquiry

7664-39-3
443 suppliers
$21.00/25ml