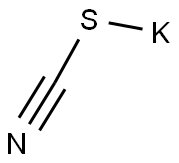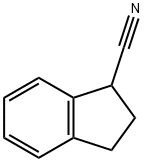
indan-1-carbonitrile synthesis
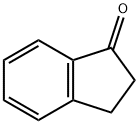
- Product Name:indan-1-carbonitrile
- CAS Number:26452-97-1
- Molecular formula:C10H9N
- Molecular Weight:143.19
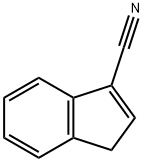
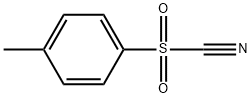
29872-81-9

26452-97-1
The general procedure for the synthesis of 2,3-dihydro-1H-indene-1-carbonitrile from 1H-indene-3-carbonitrile (CAS:29872-81-9) was as follows: 5% Pd/C catalyst was suspended in ethanol (200 mL) solution of 1H-indene-3-carbonitrile, and the reaction was carried out with vigorous stirring under hydrogen atmosphere overnight. Upon completion of the reaction, the excess hydrogen was removed, followed by filtration of the reaction mixture through a diatomaceous earth pad and concentration of the filtrate to obtain an oily material. This oily substance was redissolved in ethanol and a second filtration was performed to completely remove residual catalyst and carbon. Finally, the solvent was removed by evaporation to give 12.0108 g of 2,3-dihydro-1H-indene-1-carbonitrile in 95% yield and the product as an oil. The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-D6): δ 2.2 (m, 1H), 2.5 (m, 1H), 2.9 (m, 1H), 3.0 (m, 1H), 4.4 (t, J = 8.0 Hz, 1H), 7.2 (m, 2H), 7.3 (m, 1H), 7.4 (m, J = 7.7, 4.9 Hz, 1H).

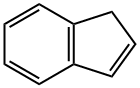
29872-81-9
0 suppliers
inquiry

26452-97-1
54 suppliers
inquiry
Yield:26452-97-1 95%
Reaction Conditions:
with hydrogen;5%-palladium/activated carbon in ethanol;
Steps:
4 Example 4; 1-indanecarbonitrile
A suspension of 5% Pd on carbon in a solution of 1 H-INDENE-3-CARBONITRILE IN ETOH (200MUT) was placed under an atmosphere of hydrogen with vigorous stirring overnight. After removal of excess hydrogen from the reaction the solution as filtered through celite and concentrated to an oil. The resulting oil was re-dissolved in EtOH and filtered a second time to remove the remaining carbon and catalyst. Evaporation of solvent afforded 12.0108g of 1-INDANECARBONITRILE (95% yield) as an oil. 1 H NMR (400 MHz, DMSO-D6) 8 ppm 2.2 (m, 1 H) 2.5 (m, 1 H) 2.9 (m, 1 H) 3.0 (m, 1 H) 4.4 (t, J=8.0 Hz, 1 H) 7.2 (m, 2 H) 7.3 (m, 1 H) 7.4 (m, J=7.7, 4.9 Hz, 1 H).
References:
WO2004/55012,2004,A1 Location in patent:Page 53
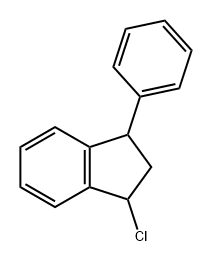
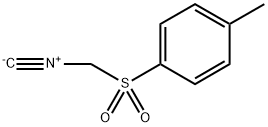
106783-39-5
0 suppliers
inquiry

26452-97-1
54 suppliers
inquiry